Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**4.2 DOSE AND METHOD OF ADMINISTRATION** **Dosage** The recommended dosage of QINLOCK is 150 mg (three 50 mg tablets) orally once daily with or without food until disease progression or unacceptable toxicity. Instruct patients to swallow tablets whole. Advise patients to take QINLOCK at the same time each day. Advise patients to take a missed dose if less than 8 hours have passed since the missed scheduled dose. Advise patients to not take the missed dose and simply resume the usual dosing schedule on the following day, if more than 8 hours have passed since the missed scheduled dose. Advise patients not to take an additional dose if vomiting occurs after taking QINLOCK and to continue with their next scheduled dose. **Dose modification guidelines** The recommended dose reduction for adverse reactions is: - QINLOCK 100 mg orally once daily. Permanently discontinue QINLOCK in patients who are unable to tolerate 100 mg orally once daily. The recommended dosage modifications of QINLOCK for adverse reactions are provided in Table 1. 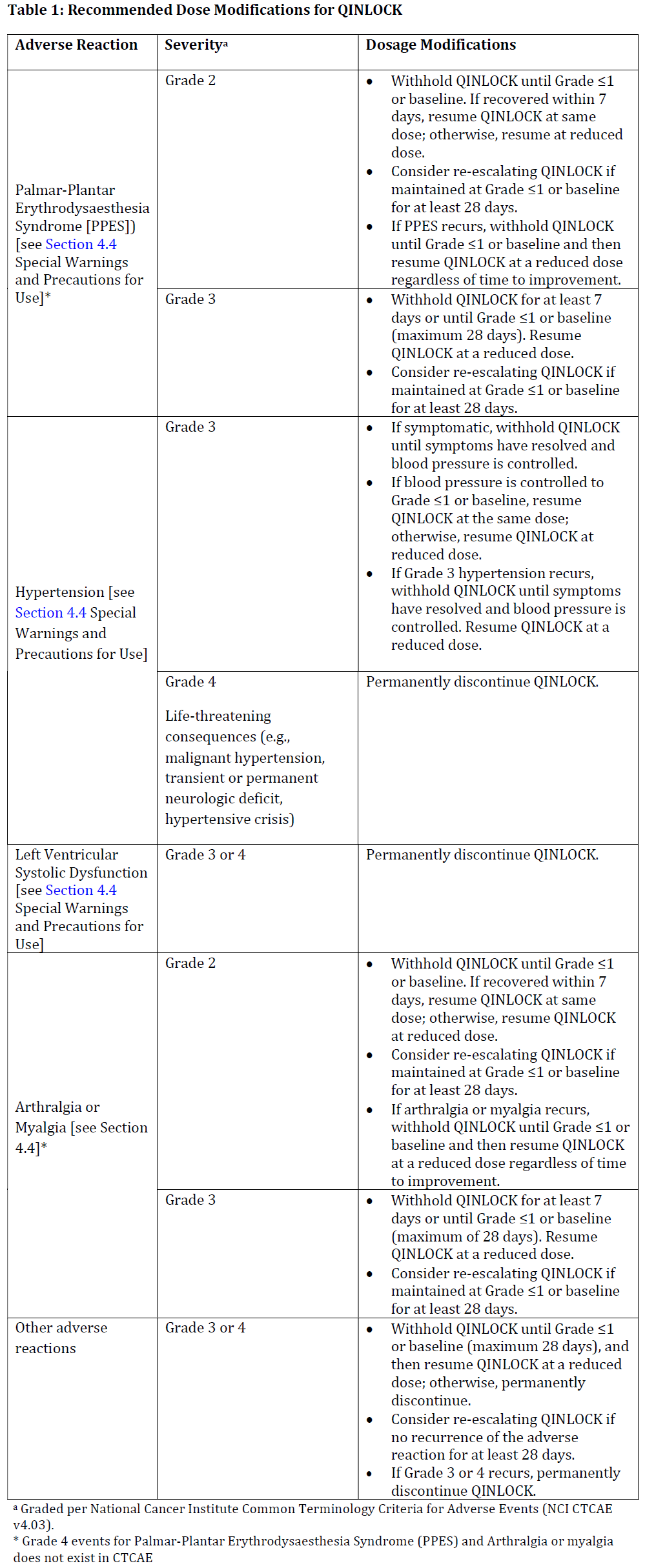 _**Paediatrics**_ The safety and effectiveness of QINLOCK in paediatric patients have not been established. _**Patients with renal impairment**_ No dose adjustment is recommended for patients with mild and moderate renal impairment \[creatinine clearance (CrCl 30 to 89 mL/min estimated by Cockcroft-Gault)\]. The pharmacokinetics and safety of QINLOCK in patients with severe renal impairment (CrCl 15 to 29 mL/min estimated by Cockcroft-Gault) have not been studied. No dosing recommendation can be made in patients with severe renal impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Patients with hepatic impairment**_ No dose adjustment is recommended in patients with mild (Child-Pugh A), moderate (Child-Pugh B) or severe (Child-Pugh C) hepatic impairment. Data in patients with severe hepatic impairment is limited, thus close monitoring of overall safety is recommended in these patients. _**Geriatrics**_ Of the 85 patients in INVICTUS who received QINLOCK 150 mg orally once daily, 24% were between 65 to 74 years of age and 9% were 75 years of age or older. In clinical studies, no clinically relevant differences were observed between elderly (aged >65 years) and younger patients (aged ≤ 65 and ≥ 18 years). **Dose Modifications for CYP3A Inducers** Avoid concomitant strong or moderate CYP3A inducers during QINLOCK treatment. If a moderate CYP3A inducer cannot be avoided, increase the QINLOCK dosing frequency from the recommended dose of 150 mg once daily to 150 mg twice daily during the co-administration period. Monitor for clinical response and tolerability. If the concomitant moderate CYP3A inducer is discontinued, resume QINLOCK dosage back to 150 mg once daily 14 days after the discontinuation of the moderate CYP3A inducer. For patients taking QINLOCK twice daily, if the patient misses a dose within 4 hours of the time it is usually taken, the patient should be instructed to take the missed dose as soon as possible and then take the next dose at the regularly scheduled time. If a patient misses a dose by more than 4 hours of the time it is usually taken, the patient should be instructed not to take the missed dose and simply resume the usual dosing schedule. Close monitoring of overall efficacy and safety is recommended in these patients \[see Section 4.5 Interactions with Other Medicines and Other Forms of Interactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\].
ORAL
Medical Information
**4.1 THERAPEUTIC INDICATIONS** QINLOCK is indicated for the treatment of adult patients with advanced gastrointestinal stromal tumours (GIST) who have received prior treatment with 3 or more kinase inhibitors, including imatinib, sunitinib, and regorafenib.
**4.3 CONTRAINDICATIONS** Use of QINLOCK is contraindicated in patients with hypersensitivity to ripretinib or to any other component of QINLOCK tablets.
L01EX19
ripretinib
Manufacturer Information
SPECIALISED THERAPEUTICS ASIA PTE. LTD.
Lonza Bend, Inc
Active Ingredients
Documents
Package Inserts
Qinlock Package Insert.pdf
Approved: April 28, 2023
