Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**Dosage and Administration** VELCADE® may be administered: - Intravenously (at a concentration of 1 mg/ml) as a 3 to 5 second bolus injection or - Subcutaneously (at a concentration of 2.5 mg/ml) Because each route of administration has a different reconstituted concentration, caution should be used when calculating the volume to be administered. At least 72 hours should elapse between consecutive doses of VELCADE®. **VELCADE® IS FOR INTRAVENOUS OR SUBCUTANEOUS USE ONLY. Intrathecal administration has resulted in death.** **Monotherapy** _**Relapsed Multiple Myeloma and Relapsed Mantle Cell Lymphoma**_ **Recommended Dosage** The recommended dose of VELCADE® is 1.3 mg/m2/dose administered twice weekly for 2 weeks (Days 1, 4, 8, and 11) followed by a 10-day rest period (Days 12–21). For extended therapy of more than 8 cycles, VELCADE® may be administered on the standard schedule or, for relapsed multiple myeloma, on a maintenance schedule of once weekly for 4 weeks (Days 1, 8, 15, and 22) followed by a 13-day rest period (Days 23 to 35) (see _Clinical Trials_ section for a description of dose administration during the trials – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). At least 72 hours should elapse between consecutive doses of VELCADE®. **Dose Modification and Re-initiation of Therapy** VELCADE® therapy should be withheld at the onset of any Grade 3 non-hematological or Grade 4 hematological toxicities excluding neuropathy as discussed below (see _Special Warnings And Special Precautions For Use_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Once the symptoms of the toxicity have resolved, VELCADE® therapy may be reinitiated at a 25% reduced dose (1.3 mg/m2/dose reduced to 1.0 mg/m2/dose; 1.0 mg/m2/dose reduced to 0.7 mg/m2/dose). **Table 1** contains the recommended dose modification for the management of patients who experience VELCADE®-related neuropathic pain and/or peripheral neuropathy. Severe autonomic neuropathy resulting in treatment interruption or discontinuation has been reported. Patients with pre-existing severe neuropathy should be treated with VELCADE® only after careful risk-benefit assessment. 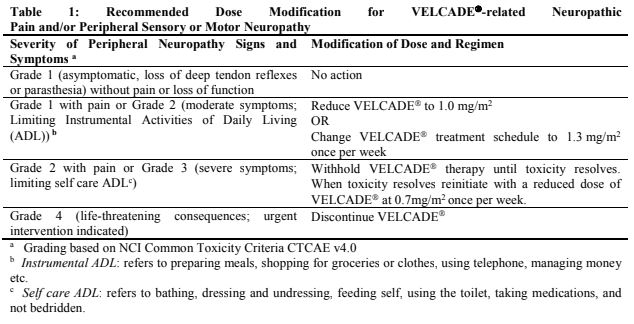 **Administration** VELCADE® is administered intravenously or subcutaneously. When administered intravenously, VELCADE® is administered as a 3–5 second bolus intravenous injection through a peripheral or central intravenous catheter followed by a flush with 0.9% sodium chloride solution for injection. For subcutaneous administration, the reconstituted solution is injected into the thighs (right or left) or abdomen (right or left). Injection sites should be rotated for successive injections. If local injection site reactions occur following VELCADE® injection subcutaneously, a less concentrated VELCADE® solution (1 mg/ml instead of 2.5 mg/ml) may be administered subcutaneously, or changed to IV injection. **Combination Therapy** **_Previously Untreated Multiple Myeloma_** **Recommended Dosage in Combination with Melphalan and Prednisone** VELCADE® (bortezomib) for Injection is administered in combination with oral melphalan and oral prednisone for nine 6-week treatment cycles as shown in **Table 2**. In Cycles 1–4, VELCADE® is administered twice weekly (days 1, 4, 8, 11, 22, 25, 29 and 32). In Cycles 5–9, VELCADE® is administered once weekly (days 1, 8, 22 and 29). At least 72 hours should elapse between consecutive doses of VELCADE®. 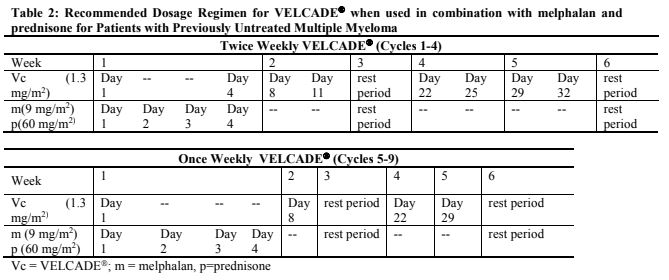 **Dose Management Guidelines for Combination Therapy with Melphalan and Prednisone** Dose modification and reinitiation of therapy when VELCADE® is administered in combination with melphalan and prednisone Prior to initiating a new cycle of therapy: - Platelet count should be ≥70 x 109/L and the absolute neutrophil count (ANC) should be ≥ 1.0 x 109/L - Non-hematological toxicities should have resolved to Grade 1 or baseline 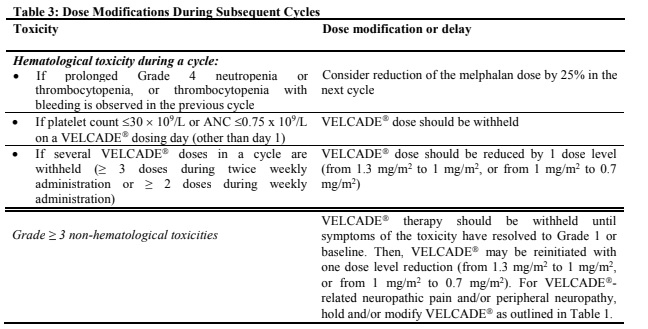 For additional information concerning melphalan and prednisone, see manufacturer's prescribing information. _**Previously Untreated Mantle Cell Lymphoma Patients Not Eligible for Haematopoietic Stem Cell Transplantation**_ **Recommended Dosage in Combination with Rituximab, Cyclophosphamide, Doxorubicin and Prednisone** For VELCADE® dosage, see Monotherapy. Six VELCADE® cycles are administered. For patients with a response first documented at Cycle 6, two additional VELCADE® cycles are recommended. The following medicinal products are administered on Day 1 of each VELCADE® 3 week treatment cycle as intravenous infusions: rituximab at 375 mg/m2, cyclophosphamide at 750 mg/m2, and doxorubicin at 50 mg/m2. Prednisone is administered orally at 100 mg/m2 on Days 1, 2, 3, 4 and 5 of each treatment cycle. **Dose Adjustments during Treatment for Patients with Previously Untreated Mantle Cell Lymphoma** Prior to the first day of each cycle (other than Cycle 1): - Platelet count should be ≥ 100 x 109/L and absolute neutrophil count (ANC) should be ≥ 1.5 x 109/L - Hemoglobin should be ≥ 8 g/dL (≥ 4.96 mmol/L) - Non-hematologic toxicity should have recovered to Grade 1 or baseline VELCADE® treatment must be withheld at the onset of any Grade 3 non-hematological or Grade 3 hematological toxicities, excluding neuropathy (see also section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For dose adjustments, see **Table 4** below. 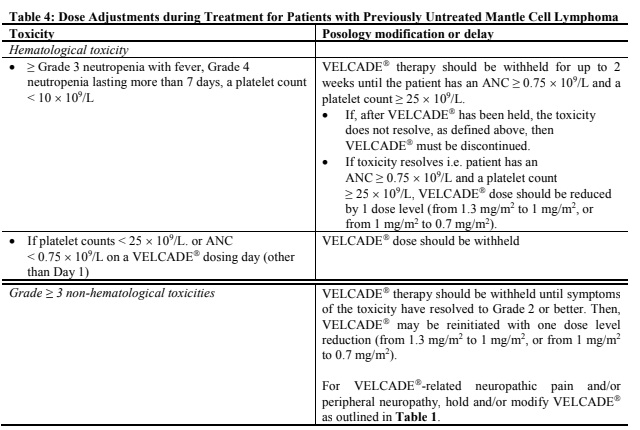 For dosing instructions for rituximab, cyclophosphamide, doxorubicin, or prednisone, see manufacturer's prescribing information. **Special Populations** **_Patients with Renal Impairment_** The pharmacokinetics of VELCADE® are not influenced by the degree of renal impairment. Therefore, dosing adjustments of VELCADE® are not necessary for patients with renal insufficiency. Since dialysis may reduce VELCADE® concentrations, the drug should be administered after the dialysis procedure (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **_Patients with Hepatic Impairment_** Patients with mild hepatic impairment do not require a starting dose adjustment and should be treated per the recommended VELCADE® dose. Patients with moderate or severe hepatic impairment should be started on VELCADE® at a reduced dose of 0.7 mg/m2 per injection during the first cycle, and a subsequent dose escalation to 1.0mg/m2 or further dose reduction to 0.5mg/m2 may be considered based on patient tolerance (see **Table 5**). 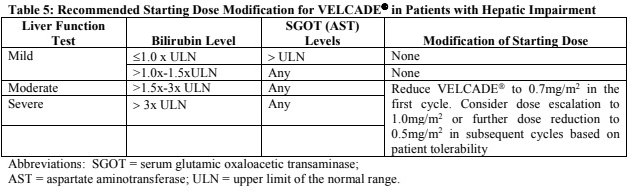
INTRAVENOUS
Medical Information
**Indications** VELCADE® (bortezomib) for Injection is indicated as part of combination therapy for the treatment of patients with previously untreated multiple myeloma. VELCADE® (bortezomib) for Injection is indicated as monotherapy for the treatment of patients with multiple myeloma who have received at least 1 prior therapy. VELCADE® (bortezomib) for Injection is indicated as monotherapy for the treatment of patients with mantle cell lymphoma who have received at least 1 prior therapy. VELCADE® (bortezomib) for Injection in combination with rituximab, cyclophosphamide, doxorubicin and prednisone is indicated for the treatment of adult patients with previously untreated mantle cell lymphoma who are unsuitable for haematopoietic stem cell transplantation.
**Contraindications** VELCADE® is contraindicated in patients with acute diffuse infiltrative pulmonary and pericardial disease and hypersensitivity to bortezomib, boron, or mannitol.
L01XX32
xl 01 xx 32
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
BSP Pharmaceuticals S.p.A. (BSP)
Fareva Pau (BULK MANUFACTURING AND PRIMARY PACKAGING)
Active Ingredients
Documents
Package Inserts
Velcade_PI.pdf
Approved: February 19, 2023
