Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**4.2 Posology and method of administration** Treatment should be initiated under the supervision of a physician experienced in the treatment of coagulation disorders. **Posology** The dosage and duration of the substitution therapy depend on the severity of the disorder, location and extent of bleeding and the patient’s clinical condition. The (functional) fibrinogen level should be determined in order to calculate individual dosage and the amount and frequency of administration should be determined on an individual patient basis by regular measurement of plasma fibrinogen level and continuous monitoring of the clinical condition of the patient and other replacement therapies used. Normal plasma fibrinogen level is in the range of 1.5–4.5 g/l. The critical plasma fibrinogen level below which haemorrhages may occur is approximately 0.5 – 1.0 g/l. In case of major surgical intervention, precise monitoring of replacement therapy by coagulation assays is essential. 1. Prophylaxis in patients with congenital hypo- or afibrinogenaemia and known bleeding tendency. To prevent excessive bleeding during surgical procedures, prophylactic treatment is recommended to raise fibrinogen levels to 1 g/l and maintain fibrinogen at this level until haemostasis is secured and above 0.5 g/l until wound healing is complete. In case of surgical procedure or treatment of a bleeding episode, the dose should be calculated as follows: 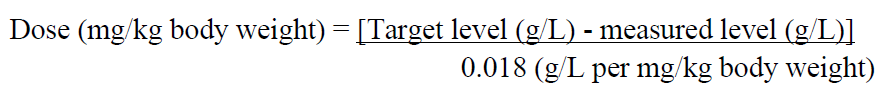 Subsequent posology (doses and frequency of injections) should be adapted based on the patient’s clinical status and laboratory results. The biological half-life of fibrinogen is 3–4 days. Thus, in the absence of consumption, repeated treatment with human fibrinogen is not usually required. Given the accumulation that occurs in case of repeated administration for a prophylactic use, the dose and the frequency should be determined according to the therapeutic goals of the physician for a given patient. Posology in specific populations **Paediatric Patients** Currently available data are described in section 4.8 and 5.1 but no recommendation on a posology can be made in children – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **Elderly patients** Clinical studies of FIBRYGA did not include patients aged 65 years and over to provide conclusive evidence as to whether or not they respond differently than younger patients. 2. Treatment of bleeding **Bleeding in patients with congenital hypo- or afibrinogenaemia** Bleeding should be treated to achieve a recommended target fibrinogen plasma level of 1 g/l. This level should be maintained until haemostasis is secured. **Bleeding in patients with acquired fibrinogen deficiency** The recommended initial dose for patients with uncontrolled severe bleeding in the course of surgical intervention is 4 g. Additional doses of 4 g are to be administered as needed to bleeding patients when FIBTEM A20 is ≤12 mm (or equivalent values generated by other thromboelastometry/thrombelastography methods). Monitor the patient’s fibrinogen plasma level or the clot firmness of the fibrin-based clot during treatment with FIBRYGA. Posology in specific populations **Paediatric Patients** There are no data available from clinical studies in this age group. No recommendation on a posology for the treatment of bleeding in acquired fibrinogen deficiency can be made in children. **Elderly patients** Clinical studies of FIBRYGA did not include sufficient numbers of patients aged 65 years and over to provide conclusive evidence as to whether or not they respond differently than younger patients. **Method of administration** Intravenous infusion or injection. FIBRYGA should be administered slowly intravenously at a recommended maximum rate of 5 mL per minute for patients with congenital hypo- or afibrinogenaemia and at a recommended maximum rate of 10 mL per minute for patients with acquired fibrinogen deficiency. For instructions on reconstitution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Treatment of bleeding and peri-operative prophylaxis in patients with congenital hypo- or afibrinogenaemia with bleeding tendency. As complementary therapy to management of uncontrolled severe haemorrhage in patients with acquired hypofibrinogenaemia in the course of surgical intervention.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
B02BB01
fibrinogen, human
Manufacturer Information
WELLCHEM PHARMACEUTICALS PTE LTD
Octapharma Pharmazeutika Produktionsges.m.b.H,(powder)
Octapharma AB (powder)
B.Braun Melsungen AG (Solvent)
Active Ingredients
Documents
Package Inserts
FIBRYGA POWDER AND SOVENT FOR SOLUTION FOR INJECTION OR INFUSION PI.pdf
Approved: June 1, 2021
