Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**4.2 Posology and method of administration** Posology Levetiracetam therapy can be initiated with either intravenous or oral administration. Conversion to or from oral to intravenous administration can be done directly without titration. The total daily dose and frequency of administration should be maintained. Levetiracetam NORMON concentrate is for intravenous use only and the recommended dose must be diluted in at least 100 ml of a compatible diluent and administered intravenously as a 15-minute intravenous infusion (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). There is no experience with administration of intravenous Levetiracetam for longer period than 4 days. Levetiracetam NORMON is an alternative for patients (adults and children from 4 years of age) when oral administration is temporarily not feasible. Please refer to Section 6.2 Incompatibilities and Section 6.6 Special precautions for disposal and other handling for recommendation on the preparation and administration of Levetiracetam NORMON 100 mg/ml concentrate for solution for infusion – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. _**Adults**_ - Monotherapy Adults and adolescents from 16 years of age The recommended starting dose is 250 mg twice daily which should be increased to an initial therapeutic dose of 500 mg twice daily after two weeks. The dose can be further increased by 250 mg twice daily every two weeks depending upon the clinical response. The maximum dose is 1500 mg twice daily. - Add-on therapy Adults (≥18 years) and adolescents (12 to 17 years) of 50 kg or more The initial therapeutic dose is 500mg twice daily. This dose can be started on the first day of treatment. Depending upon the clinical response and tolerance, the daily dose can be increased up to 1,500mg twice daily. Dose changes can be made in 500mg twice daily increases or decreases every two to four weeks. _**Children**_ The physician should prescribe the most appropriate pharmaceutical form and strength according to age, weight and dose. The safety and efficacy of Levetiracetam concentrate for solution for infusion in children less than 4 years have not been established. - Monotherapy The safety and efficacy of Levetiracetam NORMON in children and adolescents below 16 years as monotherapy treatment have not been established. No data are available. - Add-on Therapy for Children (4 to 11 years) and Adolescents (12 to 17 years) weighing less than 50 kg Levetiracetam Oral Solution is the preferred formulation for use in children under the age of 6 years. For children 6 years and above, Levetiracetam oral solution should be used for doses under 250 mg, for doses not multiple of 250 mg when dosing recommendation is not achievable by taking multiple tablets and for patients unable to swallow tablets. The initial therapeutic dose is 10 mg/kg twice daily. Depending upon the clinical response and tolerability, the dose can be increased up to 30 mg/kg twice daily. Dose changes should not exceed increases or decreases of 10 mg/kg twice daily every two weeks. The lowest effective dose should be used. Dose in children 50 kg or greater is the same as in adults. Dose recommendations for children and adolescents: 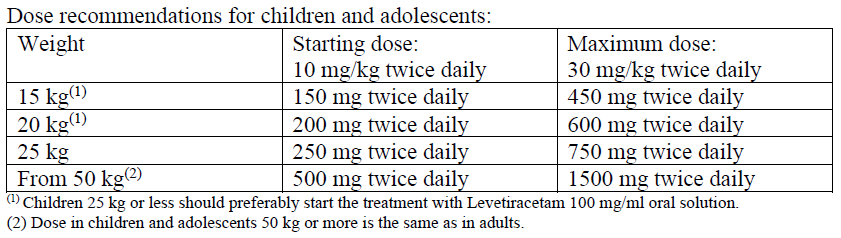 _Infants and children less than 4 years_ There are insufficient data to recommend the use of Levetiracetam Normon in children under 4 years of age. _Elderly_ Adjustment of the dose is recommended in elderly patients with compromised renal function. _Renal impairment_ The daily dose must be individualised according to renal function. (see Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) For adult patients, refer to the following table and adjust the dose as indicated. To use this dosing table, an estimate of the patient's creatinine clearance (CLcr) in ml/min is needed. The CLcr in ml/min may be estimated from serum creatinine (mg/dl) determination, for adults and adolescents weighing 50 kg or more, the following formula: 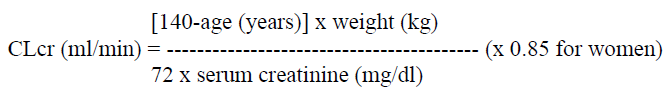 Then CLcr is adjusted for body surface area (BSA) as follows: 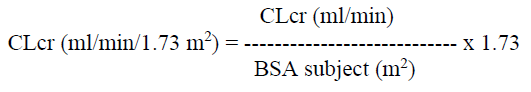 Dosing adjustment for adult and adolescent patients weighing more than 50 kg with impaired renal function: 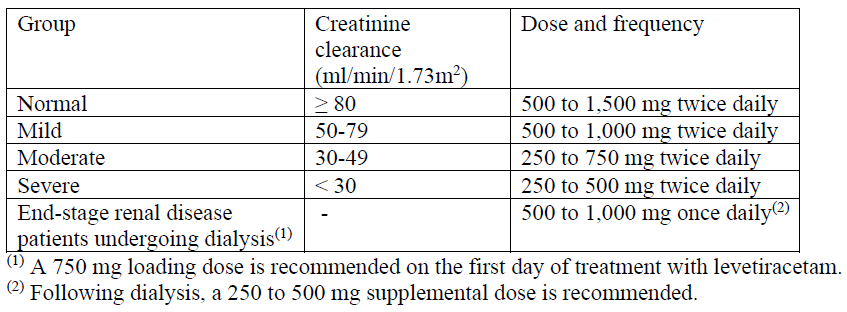 For children with renal impairment, levetiracetam dose needs to be adjusted based on the renal function as levetiracetam clearance is related to renal function. This recommendation is based on a study in adult renally-impaired patients. _Hepatic impairment_ No dose adjustment is needed in patients with mild to moderate hepatic impairment. In patients with severe hepatic impairment, the creatinine clearance may underestimate the renal insufficiency. Therefore a 50 % reduction of the daily maintenance dose is recommended when the creatinine clearance is < 60 ml/min/1.73 m2. (See Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Levetiracetam NORMON is indicated as monotherapy in the treatment of partial onset seizures with or without secondary generalisation in adults and adolescents from 16 years of age with newly diagnosed epilepsy. Levetiracetam NORMON is indicated as adjunctive therapy in the treatment of: - partial onset seizures with or without secondary generalisation in adults, adolescents and children from 4 years of age with epilepsy. - myoclonic seizures in adults and adolescents from 12 years of age with Juvenile Myoclonic Epilepsy. - primary generalised tonic-clonic seizures in adults and adolescents from 12 years of age with Idiopathic Generalised Epilepsy. Levetiracetam NORMON concentrate is an alternative for patients when oral administration is temporarily not feasible.
**4.3 Contraindications** Hypersensitivity to the active substance or other pyrrolidone derivatives or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
N03AX14
levetiracetam
Manufacturer Information
NOVEM HEALTHCARE PTE LTD
LABORATORIOS NORMON S.A.
Active Ingredients
Documents
Package Inserts
Levetiracetam NORMON_PI.pdf
Approved: March 21, 2023
