Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**4.2 Posology and method of administration** **ALK and ROS1 Testing** Detection of either ALK-positive or ROS1-positive NSCLC is necessary for selection of patients for treatment with XALKORI because these are the only patients for whom benefit has been shown. Assessment for either ALK-positive or ROS1-positive NSCLC should be performed by laboratories with demonstrated proficiency in the specific technology being utilized. Improper assay performance can lead to unreliable test results. **Recommended Dosing** The recommended dose and schedule of XALKORI is 250 mg taken orally twice daily. Continue treatment as long as the patient is deriving clinical benefit from therapy. The recommended dose of XALKORI in patients with severe renal impairment (creatinine clearance <30 mL/min) not requiring dialysis is 250 mg orally, once daily (see Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). XALKORI may be taken with or without food. Swallow capsules whole. If a dose of XALKORI is missed, make up that dose unless the next dose is due within 6 hours. If vomiting occurs after taking a dose of XALKORI, take the next dose at the regular time. **Dose Modification** Reduce dose as below for patients treated with crizotinib 250 mg orally twice daily, if one or more dose reductions are necessary, due to adverse reactions of Grade 3 or 4 severity, as defined by National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0: - First dose reduction: XALKORI 200 mg taken orally twice daily. - Second dose reduction: XALKORI 250 mg taken orally once daily. - Permanently discontinue if unable to tolerate XALKORI 250 mg taken once daily. Dose reduction guidelines are provided in Tables 1 and 2. For patients treated with a lower dose of crizotinib than 250 mg twice daily, then use the recommendations in Table 1 and Table 2 accordingly. 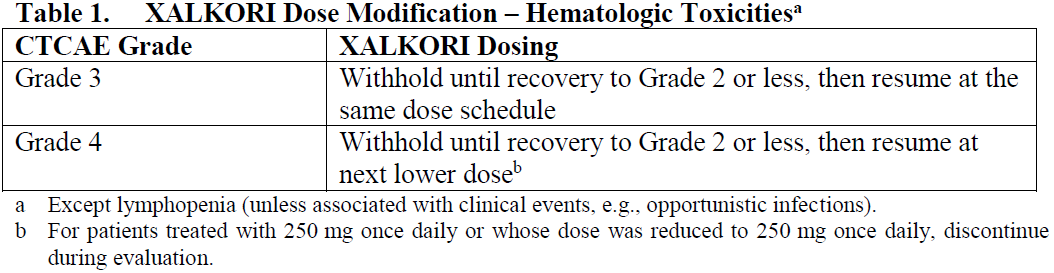 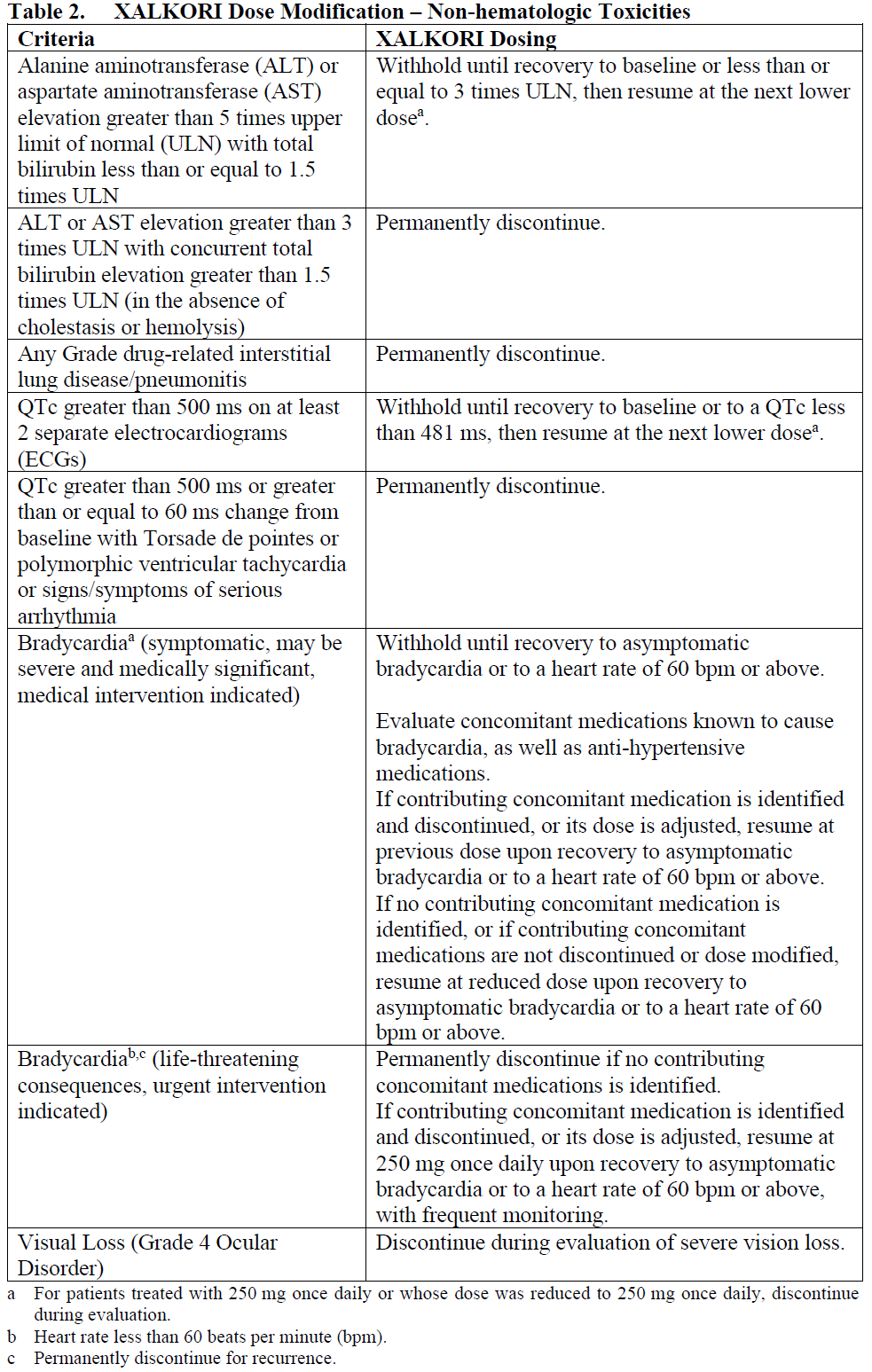 Monitor complete blood counts including differential white blood cell counts monthly and as clinically indicated, with more frequent repeat testing if Grade 3 or 4 abnormalities are observed, or if fever or infection occurs.
ORAL
Medical Information
**4.1 Therapeutic indications** XALKORI is indicated for the treatment of patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) that is anaplastic lymphoma kinase (ALK)-positive as detected by an accurate and validated assay. XALKORI is indicated for the treatment of patients with locally advanced or metastatic NSCLC that is ROS1-positive as detected by an accurate and validated assay.
**4.3 Contraindications** Use of XALKORI is contraindicated in patients with hypersensitivity to crizotinib or to any of the excipients.
L01XE16
xl 01 xe 16
Manufacturer Information
PFIZER PRIVATE LIMITED
Pfizer Manufacturing Deutschland GmbH
Active Ingredients
Documents
Package Inserts
Xalkori Capsules PI.pdf
Approved: January 31, 2023
