Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Therapy should be initiated by a physician experienced in the management of chronic hepatitis B infection. Posology _Compensated liver disease_ The recommended dose of entecavir for chronic hepatitis B virus infection in nucleoside-treatment-naïve adults and adolescents 16 years of age and older is 0.5 mg once daily, with or without food. The recommended dose of entecavir in adults and adolescents (≥16 years of age) with a history of hepatitis B viremia while receiving lamivudine or known lamivudine resistance mutations is 1 mg once daily, which must be taken on an empty stomach (at least 2 hours after a meal and 2 hours before the next meal). _Decompensated liver disease_ The recommended dose of entecavir for chronic hepatitis B virus infection in adults with decompensated liver disease is 1 mg once daily, which must be taken on an empty stomach (at least 2 hours after a meal and 2 hours before the next meal). _Renal impairment_ In subjects with renal impairment, the apparent oral clearance of entecavir decreased as creatinine clearance decreased. Dosage adjustment is recommended for patients with creatinine clearance less than 50 mL/min, including patients on hemodialysis or continuous ambulatory peritoneal dialysis (CAPD), as shown in Table 1. The once-daily dosing regimens are preferred. 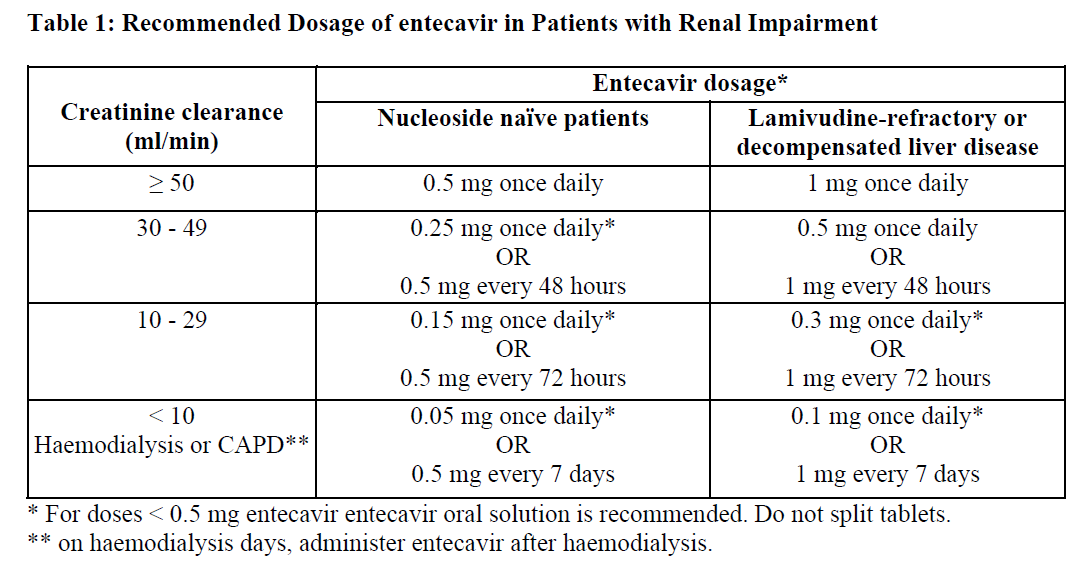 _Hepatic impairment_ No dose adjustment is required in patients with hepatic impairment. Method of administration Oral use. Duration of Therapy The optimal duration of treatment with entecavir for patients with chronic hepatitis B virus infection and the relationship between treatment and long-term outcomes such as cirrhosis and hepatocellular carcinoma are unknown.
ORAL
Medical Information
**4.1. Therapeutic indications** Adult indication Treatment of chronic hepatitis B virus (HBV) infection (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) in adults with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease. The following points should be considered when initiating therapy with entecavir: - This indication is based on histologic, virologic, biochemical, and serologic responses in nucleoside-treatment-naïve and lamivudine-resistant adult subjects with HBeAg-positive or HBeAg-negative chronic HBV infection with compensated liver disease. - Virologic, biochemical, serologic, and safety data are available from a controlled study in adult subjects with chronic HBV infection and decompensated liver disease. - Virologic, biochemical, serologic, and safety data are available for a limited number of adult subjects with HIV/HBV co-infection who have received prior lamivudine therapy.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
J05AF10
entecavir
Manufacturer Information
LOTUS INTERNATIONAL PTE. LTD.
Sanofi Ilac Sanayi ve Ticaret Anonim Sirketi
MEDIS INTERNATIONAL a.s., výrobní závod Bolatice (Primary and Secondary packager)
Active Ingredients
Documents
Package Inserts
Entecavir Alvogen PI.pdf
Approved: April 6, 2021
