Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** Monitor carefully patients for signs and symptoms of hypersensitivity reactions during and following each administration of Ferinject. Ferinject should only be administered when staff trained to evaluate and manage anaphylactic reactions is immediately available, in an environment where full resuscitation facilities can be assured. The patient should be closely observed for signs and sympotms of hypersensitivity reactions during and for at least 30 minutes following each Ferinject administration. Posology The posology of Ferinject follows a stepwise approach: \[1\] determination of the individual iron need, \[2\] calculation and administration of the iron dose(s), and \[3\] post-iron repletion assessments. These steps are outlined below: _Step 1: Determination of the iron need_ The individual iron need for repletion using Ferinject is determined based on the patient’s body weight and haemoglobin (Hb) level. Refer to Table 1 for determination of the iron need: 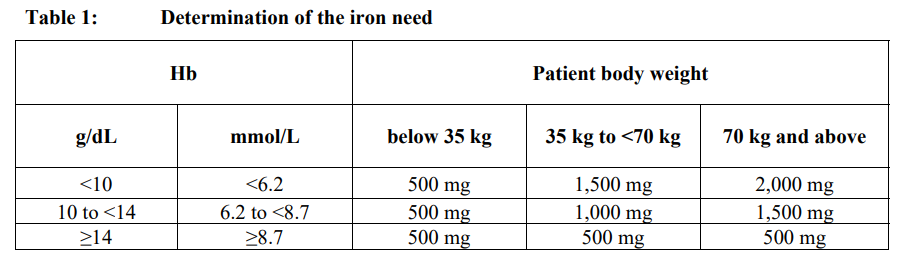 Iron deficiency must be confirmed by laboratory tests as stated in 4.1. _Step 2: Calculation and administration of the maximum individual iron dose(s)_ Based on the iron need determined above the appropriate dose(s) of Ferinject should be administered taking into consideration the following: A single Ferinject administration should not exceed: - 15 mg iron/kg body weight (for administration by intravenous injection) or 20 mg iron/kg body weight (for administration by intravenous infusion) - 1,000 mg of iron (20 mL Ferinject) The maximum recommended cumulative dose of Ferinject is 1,000 mg of iron (20 mL Ferinject) per week. _Step 3: Post-iron repletion assessments_ Re-assessment should be performed by the clinician based on the individual patient’s condition. The Hb level should be re-assessed no earlier than 4 weeks post final Ferinject administration to allow adequate time for erythropoiesis and iron utilisation. In the event the patient requires further iron repletion, the iron need should be recalculated using Table 1 above. (See section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Special Population – patients with haemodialysis-dependent chronic kidney disease_ A single maximum daily dose of 200 mg iron should not be exceeded in haemodialysis-dependent chronic kidney disease patients (see also section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population_ The use of Ferinject has not been studied in children, and therefore is not recommended in children under 14 years. Method of administration Ferinject must only be administered by the intravenous route: - by injection, or - by infusion, or - during a haemodialysis session undiluted directly into the venous limb of the dialyser. Ferinject must not be administered by the subcutaneous or intramuscular route. _Intravenous Injection_ Ferinject must be administered only by the intravenous injection using undiluted solution. The maximum single dose is 15 mg iron/kg body weight but should not exceed 1,000 mg iron. The administration rates are as shown in Table 2: 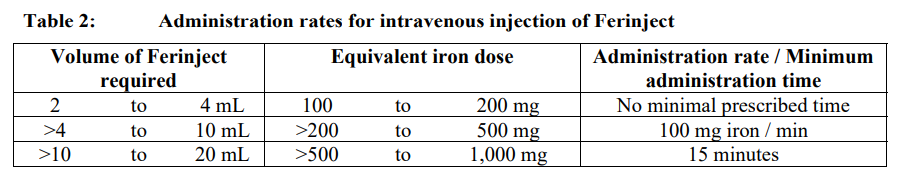 _Intravenous infusion_ Ferinject may be administered by intravenous infusion, in which case it must be diluted. The maximum single dose is 20 mg iron/kg body weight, but should not exceed 1,000 mg iron. For infusion, Ferinject must only be diluted in sterile 0.9% m/V sodium chloride solution as shown in Table 3. Note: for stability reasons, Ferinject should not be diluted to concentrations less than 2 mg iron/mL (not including the volume of the ferric carboxymaltose solution). For further instructions on dilution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. 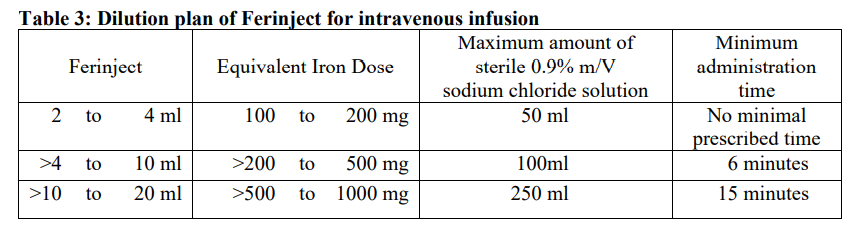
INTRAVENOUS DRIP, INTRAVENOUS BOLUS
Medical Information
**4.1 Therapeutic indications** Ferinject is indicated for the treatment of iron deficiency when (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - oral iron preparations are ineffective. - oral iron preparations cannot be used. - there is a clinical need to deliver iron rapidly. The diagnosis must be based on laboratory tests.
**4.3 Contraindications** The use of Ferinject is contraindicated in cases of: - Hypersensitivity to ferric carboxymaltose complex, to ferric carboxymaltose solution or to any of its excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ - Anaemia not attributed to iron deficiency, e.g. other microcytic anaemia - Evidence of iron overload or disturbances in utilisation of iron
B03AC01
xb 03 ac 01
Manufacturer Information
VIFOR PHARMA ASIA PACIFIC PTE. LTD.
IDT Biologika GmbH
Active Ingredients
Documents
Package Inserts
Ferinject Solution for Injection PI.pdf
Approved: January 14, 2022
