Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
INTRAVASCULAR
Medical Information
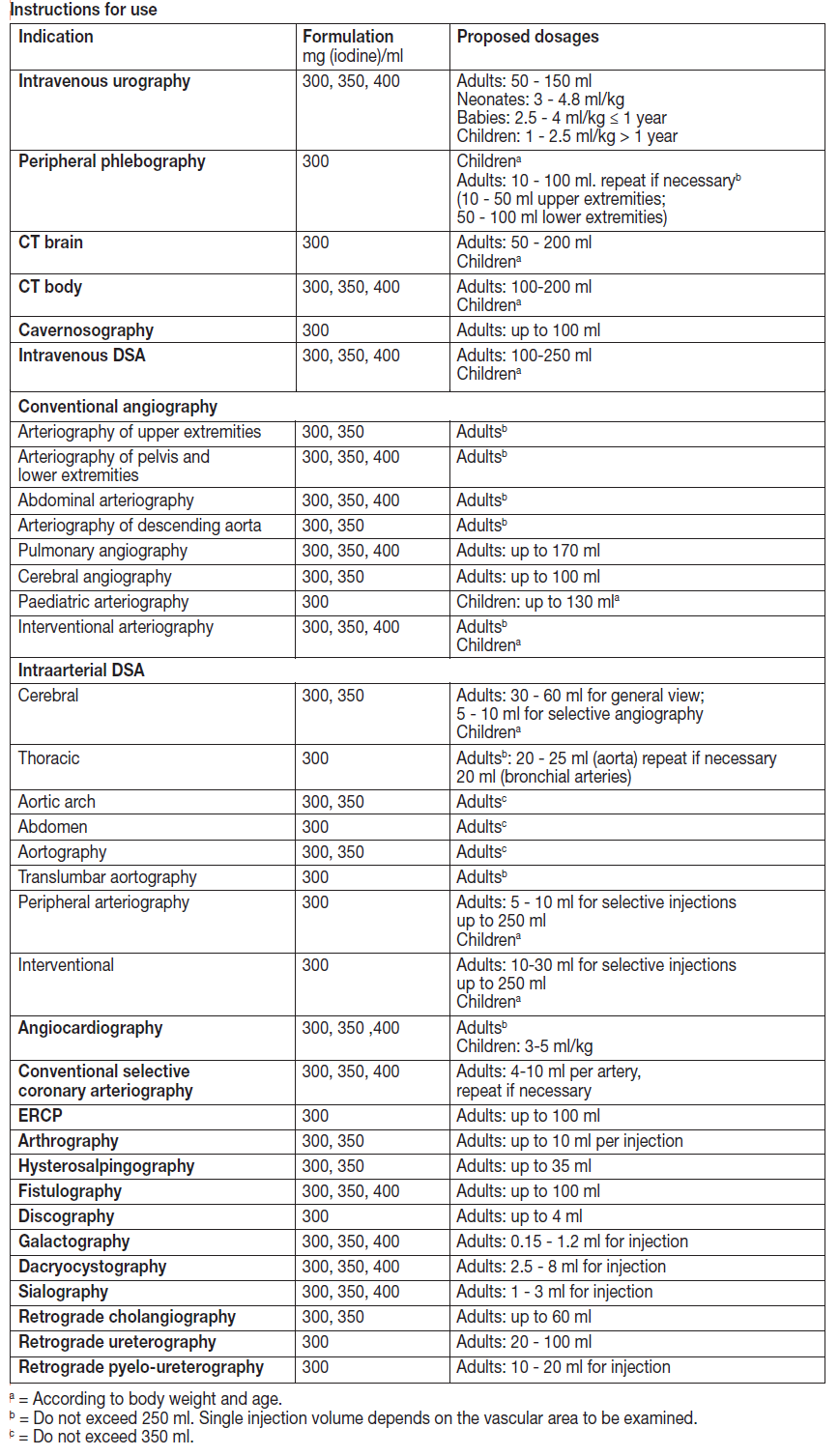
**Indications** Iomeron 300Intravenous urography (in adults and paediatrics), peripheral phlebography, CT (brain and body), cavernosography, intravenous DSA, conventional angiography, intraarterial DSA, angiocardiography (in adults and paediatrics), conventional selective coronary arteriography, interventional coronary arteriography, ERCP, arthrography, hysterosalpingography, fistulography, discography, galactography, cholangiography, dacryocystography, sialography, retrograde urethrography, retrograde pyelo-ureterography.Iomeron 350Intravenous urography (in adults and paediatrics), CT (body), intravenous DSA, conventional angiography, intraarterial DSA, angiocardiography (in adults and paediatrics), conventional selective coronary arteriography, interventional coronarography, arthrography, hysterosalpingography, fistulography, galactography, cholangiography, dacryocystography, sialography.Iomeron 400Intravenous urography, (in adults including those with renal impairment or diabetes), CT (body), conventional angiography, intraarterial DSA, angiocardiography (in adults and paediatrics), conventional selective coronary arteriography, interventional coronary arteriography, fistulography, galactography, dacryocystography, sialography. CT: Computed Tomography DSA: Digital Subtraction Angiography ERCP: Endoscopic Retrograde Cholangio-Pancreatography
**Contraindications** Iomeprol Injection should not be administered to patients with known hypersensitivity to iomeprol or any of the excipients. Investigations of the female genitalia are contraindicated in suspected or confirmed pregnancy and in cases of acute inflammation.
V08AB10
iomeprol
Manufacturer Information
DCH AURIGA SINGAPORE
Patheon Italia S.p.A
Active Ingredients
Documents
Package Inserts
Iomeron Injection PI.pdf
Approved: June 8, 2023
