Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** Dosage in Adults with Creatinine Clearance (CrCl >50 mL/min) The recommended dosage of Zavicefta in adults is 1 vial where each vial contains 2 g ceftazidime and 0.5 g avibactam administered by intravenous (IV) infusion over 2 hours. Treatment is repeated every 8 hours. The duration of treatment is provided in Table 1. Treatment Duration for Adult Patients 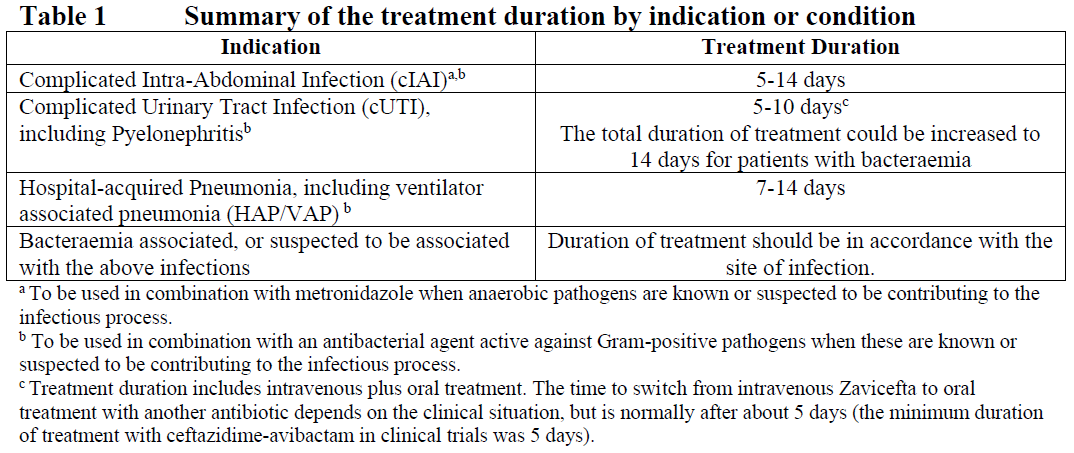 The duration of treatment should be guided by the severity of the infection, the pathogen(s) and the patient’s clinical and bacteriological progress. Dosage in paediatric patients with creatinine clearance (CrCl) >50 mL/min/1.73 m2 The recommended dosage of Zavicefta in paediatric patients (3 months to <18 years) is based on the age and weight of the patient. Zavicefta is administered every 8 hours by intravenous infusion over 2 hours, see Table 2 (see also sections 4.4 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The duration of therapy should be guided by the severity, site of infection and the patient’s clinical and bacteriological progress. 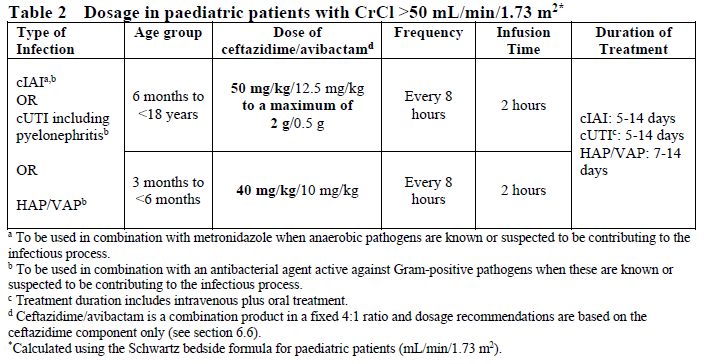 **Special populations** **Elderly patients** No dosage adjustment is considered necessary in elderly patients (≥65 years). The dose regimen should be adjusted if renal impairment is present (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Patients with renal impairment** The following dose adjustment is recommended in patients with renal impairment (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Dose adjustments for Zavicefta for patients with an estimated creatinine clearance (CrCl) ≤50 mL/min are outlined in Table 3 below. _Dosage in adults and paediatric patients with creatinine clearance (CrCl) ≤50 mL/min_ 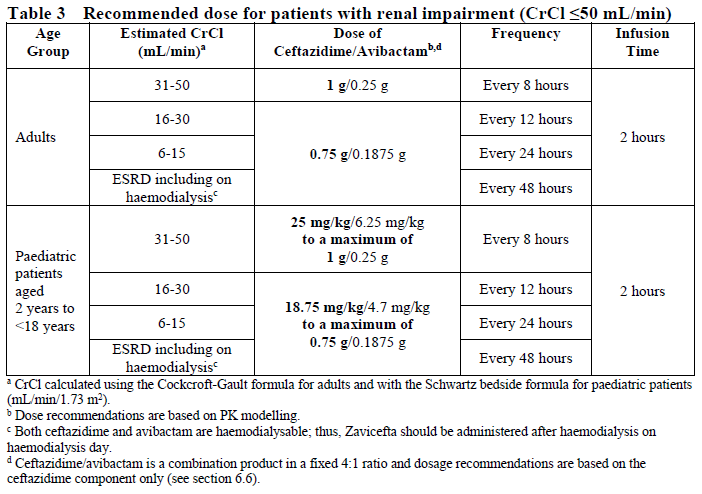 _Dosage in paediatric patients <2 years of age with creatinine clearance (CrCl) ≤50 mL/min/1.73 m2_ 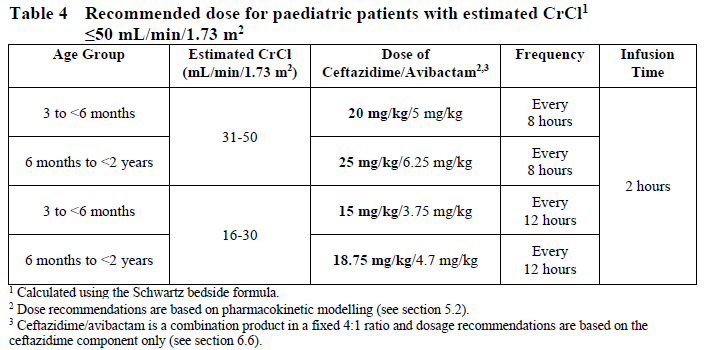 There is insufficient information to recommend a dosage regimen for paediatric patients <2 years of age that have a CrCl <16 mL/min/1.73 m2. In patients with impaired renal function, regular monitoring of estimated creatinine clearance is advised as in some patients, especially early in the course of their infection, the creatinine clearance estimated from serum creatinine can change quickly. **Haemodialysis** Both ceftazidime and avibactam are haemodialysable; thus, Zavicefta should be administered after haemodialysis on haemodialysis day. **Haemofiltration** There is insufficient data to make specific dosage adjustment recommendations for patients undergoing continuous veno-venous haemofiltration. **Peritoneal dialysis** There is insufficient data to make specific dosage adjustment recommendations for patients undergoing peritoneal dialysis. **Patients with hepatic impairment** No dosage adjustment is considered necessary in patients with hepatic impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Close clinical monitoring for safety and efficacy is advised. **Paediatric patients** Safety and efficacy in paediatric patients <18 years of age have not been established for HAP/VAP and is based on extrapolation (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Safety and efficacy in paediatric patients <3 months old have not been established. **Method of administration** Zavicefta is administered to adults by intravenous infusion over 2 hours in an appropriate infusion volume (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For paediatric patients, the infusion volume may be adjusted (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Constitution and compatibility** For instructions on reconstitution and dilution of the medicinal product before administration see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Zavicefta is indicated in adults, infants (aged 3 months and older), children, and adolescents for the treatment of the following infections (see sections 4.4 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_): - Complicated Intra-Abdominal Infection (cIAI) - Complicated Urinary Tract Infection (cUTI), including pyelonephritis - Hospital-acquired Pneumonia (HAP), including ventilator associated pneumonia (VAP) Treatment of adult patients with bacteraemia that occurs in association with, or is suspected to be associated with cIAI, cUTI, or HAP/VAP. Consideration should be given to official guidance on the appropriate use of antibacterial agents. For treatment of cIAI use in combination with metronidazole.
**4.3 Contraindications** Hypersensitivity to the active substances or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Hypersensitivity to the cephalosporin class of antibacterials. Immediate and severe hypersensitivity (e.g. anaphylactic reaction) to any other type of β-lactam antibacterial agent (e.g. penicillins, monobactams or carbapenems).
J01DD52
ceftazidime and beta-lactamase inhibitor
Manufacturer Information
PFIZER PRIVATE LIMITED
ACS Dobfar S.p.A.
ACS Dobfar SpA (Sterile ceftazidime carbonate blend) (Drug Product Intermediate)
Active Ingredients
Documents
Package Inserts
Zavicefta PI.pdf
Approved: March 20, 2023
