Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
**4.2 Posology and method of administration** **Schizophrenia** **Usual dose** The recommended starting and target dose for aripiprazole is 10 or 15 mg/day administered on a once-a-day schedule without regard to meals. Aripiprazole has been systematically evaluated and shown to be effective in a dose range of 10 to 30 mg/day, however, doses higher than 10 or 15 mg/day were not more effective than 10 or 15 mg/day. Dosage increases should generally not be made before 2 weeks, the time needed to achieve steady state. **Maintenance Therapy** While there is no body of evidence available to answer the question of how long a patient treated with aripiprazole should remain on it, systematic evaluation of patients with schizophrenia who had been symptomatically stable on other antipsychotic medication, for periods of 3 months or longer, were discontinued from those medications, and were then administered aripiprazole 15 mg/day and observed for relapse during a period of up to 26 weeks, demonstrated a benefit of such maintenance treatment. Patients should be periodically reassessed to determine the need for maintenance treatment. **Switching from Other Antipsychotics** There are no systematically collected data to specifically address switching patients with schizophrenia from other antipsychotics to aripiprazole or concerning concomitant administration with other antipsychotics. While immediate discontinuation of the previous antipsychotic treatment may be acceptable for some patients with schizophrenia, more gradual discontinuation may be most appropriate for others. In all cases, the period of overlapping antipsychotic administration should be minimized. **Bipolar Disorder** **Acute Treatment of Manic and Mixed Episodes** The recommended starting dose for aripiprazole is 15 mg given once daily as monotherapy and 10 to 15 mg given once daily as adjunctive therapy with lithium or valproate. Aripiprazole can be given without regard to meals. The recommended target dose of aripiprazole is 15 mg/day, as monotherapy or as adjunctive therapy with lithium or valproate. The dose may be increased to 30 mg/day based on clinical response. The safety of doses above 30 mg/day has not been evaluated in clinical trials. **Maintenance Therapy** The recommended dose for maintenance treatment is the same dose needed to stabilize patients during acute treatment for adult patients. Systematic evaluation of adult patients with Bipolar I Disorder experiencing a manic or mixed episode, who had been symptomatically stable on aripiprazole tablets (15 mg/day or 30 mg/day with a starting dose of 30 mg/day) for 6 consecutive weeks and then randomized to Aripiprazole Tablets (15 mg/day or 30 mg/day) or placebo for at least 6 months and up to an additional 17 months of observation for relapse, demonstrated a benefit of such maintenance treatment. Patients should be periodically reassessed to determine the need for maintenance treatment. **Hepatic and Renal impairment** No dosage adjustment for aripiprazole is required on the basis of a patient’s hepatic function (mild to severe hepatic impairment, Child-Pugh score between 5 and 15), or renal function (mild to severe renal impairment, glomerular filtration rate between 15 and 90 mL/minute). **Elderly** No dosage adjustment is recommended for elderly patients. Aripiprazole is not approved for treatment of patients with psychosis associated with Alzheimer's disease. **Other Specific Populations** No dosage adjustment for aripiprazole is required on the basis of a patient's sex, race, or smoking status. **Dosage Adjustments for Cytochrome P450 Considerations** Dosage adjustments are recommended in patients who are known CYP2D6 poor metabolizers and in patients taking concomitant CYP3A4 inhibitors or CYP2D6 inhibitors or strong CYP3A4 inducers (see Table 1). When the co-administered drug is withdrawn from the combination therapy, aripiprazole dosage should then be adjusted to its original level. When the co-administered CYP3A4 inducer is withdrawn, aripiprazole dosage should be reduced to the original level over 1 to 2 weeks. Patients who may be receiving a combination of strong, moderate, and weak inhibitors of CYP3A4 and CYP2D6 (e.g., a strong CYP3A4 inhibitor and a moderate CYP2D6 inhibitor or a moderate CYP3A4 inhibitor with a moderate CYP2D6 inhibitor), the dosing may be reduced to one-quarter (25%) of the usual dose initially and then adjusted to achieve a favourable clinical response. 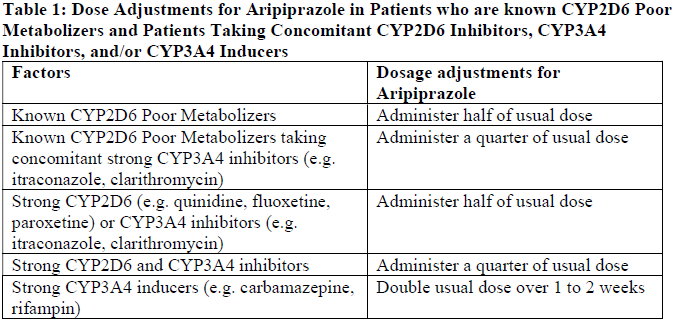 **CYP2D6 Poor Metabolizers** Dosage adjustment is recommended in known CYP2D6 poor metabolizers due to high aripiprazole concentrations. Approximately 8% of Caucasians and 3 to 8% of Black/African Americans cannot metabolize CYP2D6 substrates and are classified as poor metabolizers (PM). The rest are extensive metabolizers (EM). PMs have about an 80% increase in aripiprazole exposure and about a 30% decrease in exposure to the active metabolite compared to EMs, resulting in about a 60% higher exposure to the total active moieties from a given dose of aripiprazole compared to EMs. Co-administration of Aripiprazole with known inhibitors of CYP2D6, such as quinidine or fluoxetine in EMs, approximately doubles aripiprazole plasma exposure, and dose adjustment is needed. Similarly, PMs have higher exposure to aripiprazole compared to EMs; hence, PMs should have their initial dose reduced by one-half. Laboratory tests are available to identify CYP2D6 PMs. Aripiprazole does not inhibit or induce the CYP2D6 pathway. **Method of administration** Aripdon is for oral use.
ORAL
Medical Information
**4.1 Therapeutic indications** ARIPDON is indicated for the treatment of schizophrenia. The efficacy of aripiprazole in the treatment of schizophrenia was established in four short-term (4- and 6-week) controlled trials in adults. Maintenance efficacy was demonstrated in one trial in adults. The physician who elects to use aripiprazole for extended periods should periodically re-evaluate the long- term usefulness of the drug for the individual patient. ARIPDON is indicated for the treatment of acute manic and mixed episodes associated with Bipolar I Disorder and for maintaining stability or preventing recurrence, as monotherapy in adults,and as an adjunct to lithium or valproate in adults. The efficacy of aripiprazole as monotherapy was established in four 3-week monotherapy trials in adults. Efficacy as adjunctive therapy was established in one 6-week adjunctive trial in adults. Maintenance efficacy was demonstrated in one monotherapy maintenance trial and in one adjunctive maintenance trial in adults. Physicians who elect to use ARIPDON for extended periods, should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
**4.3 Contraindications** Aripiprazole is contraindicated in patients with a history of a hypersensitivity reaction to aripiprazole. Reactions have ranged from pruritus/urticaria to anaphylaxis.
N05AX12
aripiprazole
Manufacturer Information
MANSA HEALTHCARE PTE. LTD.
PHARMATHEN INTERNATIONAL S.A
Pharmathen S.A. (Primary and Secondary Packager)
Active Ingredients
Documents
Package Inserts
Aripdon Tablet PI.pdf
Approved: February 13, 2023
