Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**Posology and method of administration** The dosage may vary depending on the type of examination, the age, weight, cardiac output and general condition of the patient and the technique used. Usually approximately the same iodine concentration and volume is used as with other iodinated X-ray contrast media in current use, but adequate diagnostic information has also been obtained in some studies with iodixanol injection with somewhat lower iodine concentration. Adequate hydration should be assured before and after administration as for other contrast media. The product is for intravenous, intra-arterial and intrathecal use. The following dosages may serve as a guide. The doses given for intra-arterial use are for single injections that may be repeated. 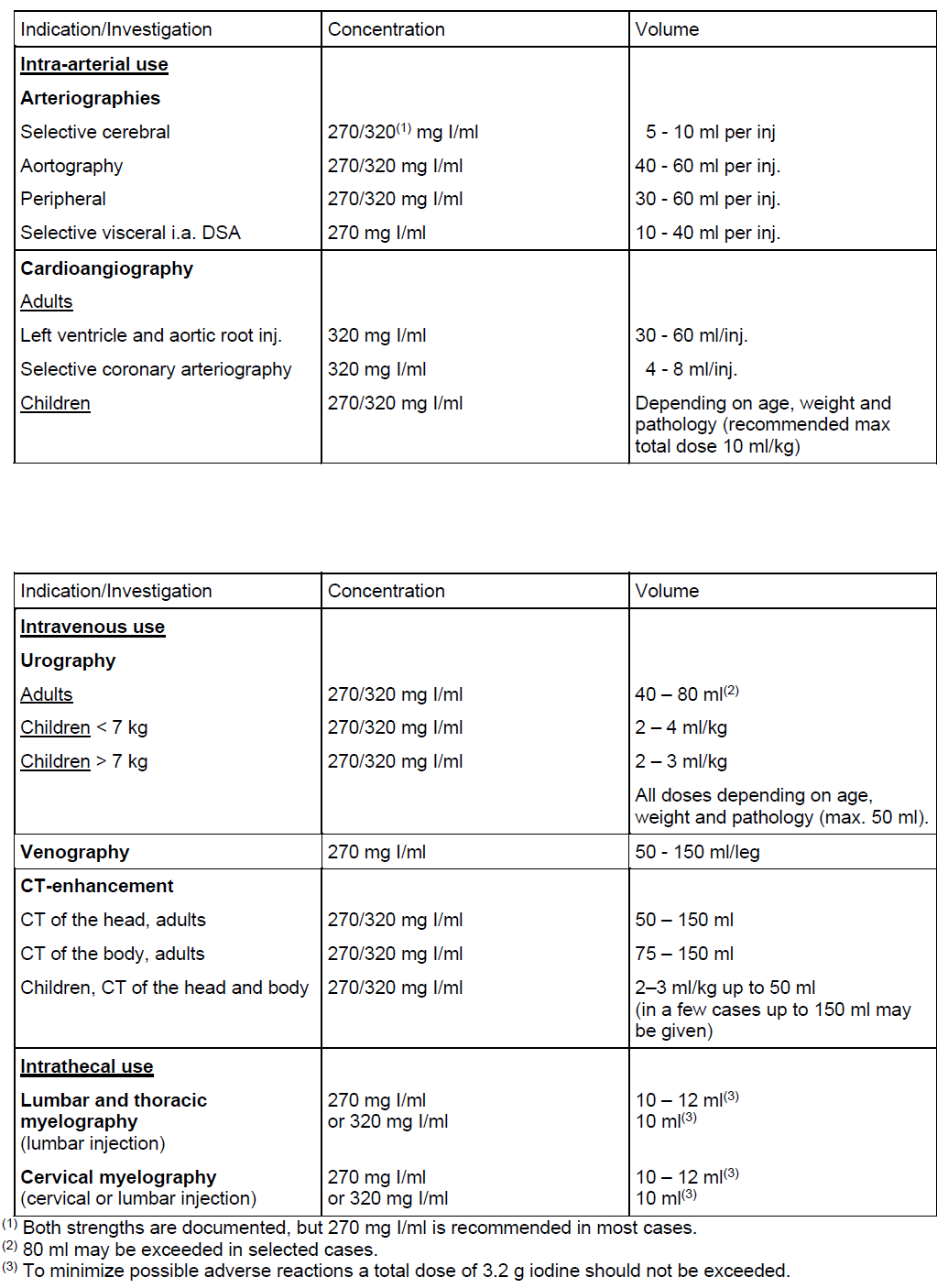 **Elderly: As for other adults**
INTRAVENOUS, INTRA-ARTERIAL, INTRATHECAL
Medical Information
**Indications** X-ray contrast medium for cardioangiography, cerebral angiography (conventional), peripheral arteriography (conventional), abdominal angiography (i.a. DSA), urography, venography, CT- enhancement. Lumbar, thoracic and cervical myelography.
**Contraindications** Hypersensitivity to the active substance or to any of the excipients. Manifest thyrotoxicosis.
V08AB09
iodixanol
Manufacturer Information
GE HEALTHCARE PTE. LTD.
GE HEALTHCARE IRELAND LIMITED (by Parametric Release)
GE HEALTHCARE AS (by Parametric Release)
GE HEALTHCARE (SHANGHAI) CO., LTD
Active Ingredients
Documents
Package Inserts
Visipaque Injection PI.pdf
Approved: November 19, 2020
