Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** Sorafenib treatment should be supervised by a physician experienced in the use of anticancer therapies. Posology _Recommended dose_ The recommended daily dose of sorafenib is 400 mg (2 x 200 mg tablets) taken twice a day, either without food or together with a moderate fat meal. _Method of administration_ For oral use. To be swallowed with a glass of water. _Duration of treatment_ Treatment should be continued until the patient is no longer clinically benefiting from therapy or until unacceptable toxicity occurs. Dose titration, dose adjustment, special monitoring advice _Dose Reduction for Hepatocellular Carcinoma and advanced Renal cell Carcinoma_ Management of suspected adverse drug reactions may require temporary interruption and/or dose reduction of sorafenib therapy. When dose reduction is necessary during the treatment of hepatocellular carcinoma (HCC) and advanced renal cell carcinoma (RCC), the sorafenib dose should be reduced to two tablets of 200 mg once daily (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Dose Reduction for Differentiated Thyroid Carcinoma Management of suspected adverse drug reactions may require temporary interruption and/or dose reduction of sorafenib therapy. When dose reduction is necessary during the treatment of differentiated thyroid carcinoma, the sorafenib dose should be reduced to 600mg daily in divided doses (two tablets of 200mg and one tablet of 200mg twelve hours apart). If additional dose reduction is necessary, sorafenib may be reduced to one tablet of 200mg twice daily, followed by one tablet of 200mg once daily. After improvement of non-hematological adverse reactions, the dose of sorafenib may be increased. 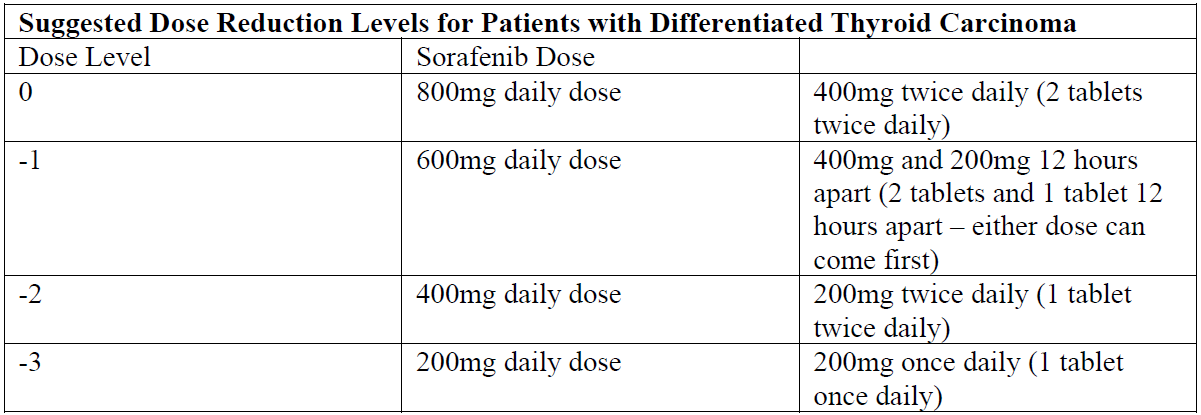 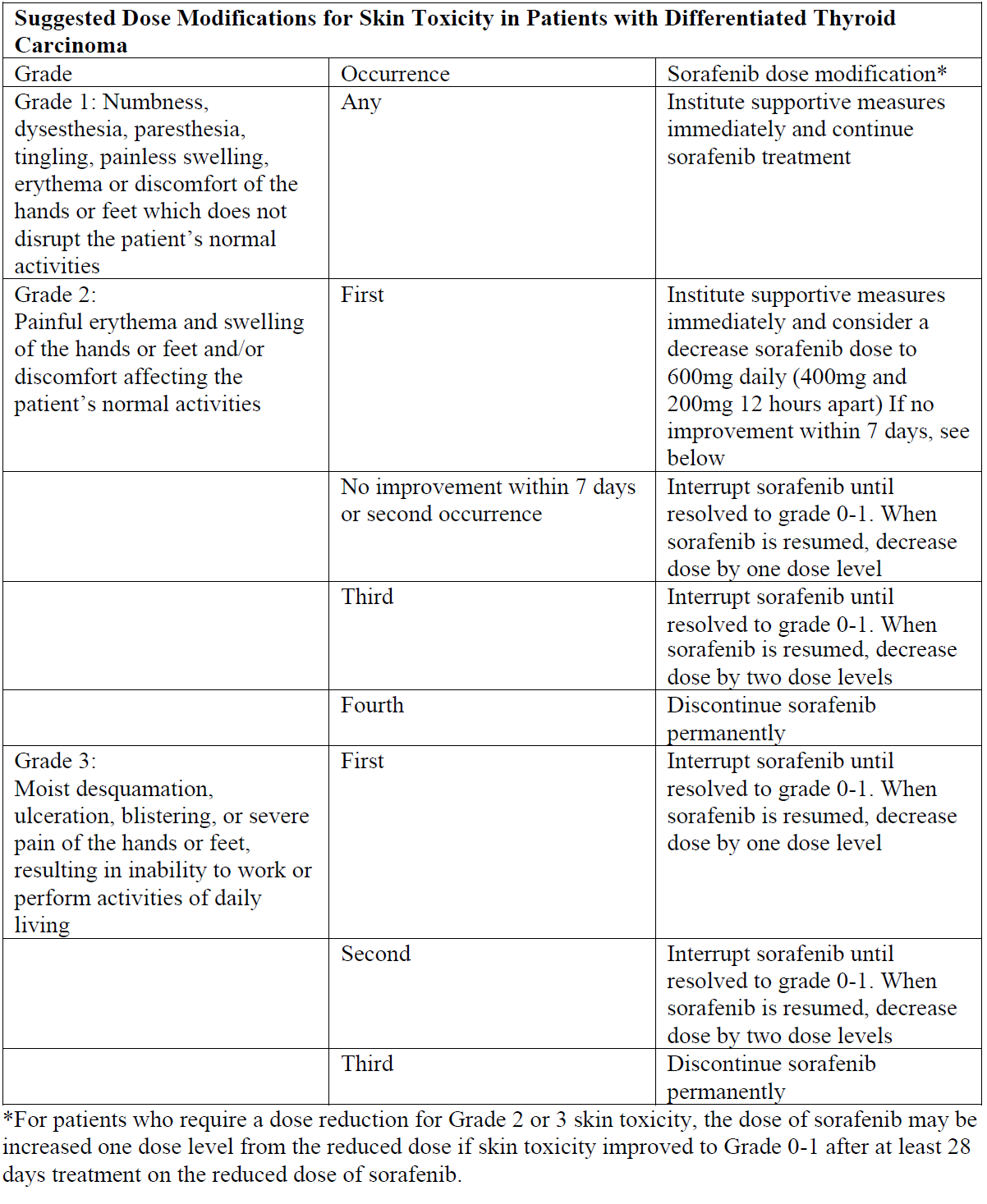 _Paediatric population_ The safety and efficacy of sorafenib in children and adolescents aged < 18 years have not yet been established. No data are available. _Elderly population_ No dose adjustment is required on the basis of patient age (above 65 years), gender, or body weight. _Renal impairment_ No dose adjustment is required in patients with mild, moderate, or severe renal impairment not requiring dialysis. Sorafenib has not been studied in patients undergoing dialysis (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Monitoring of fluid balance and electrolytes in patients at risk of renal dysfunction is advised. _Hepatic impairment_ No dose adjustment is required in patients with Child Pugh A or B (mild to moderate) hepatic impairment. No data is available on patients with Child Pugh C (severe) hepatic impairment (see sections 4.4 and 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Method of administration For oral use. It is recommended that sorafenib should be administered without food or with a low or moderate fat meal. If the patient intends to have a high-fat meal, sorafenib tablets should be taken at least 1 hour before or 2 hours after the meal. The tablets should be swallowed with a glass of water.
ORAL
Medical Information
**4.1 Therapeutic indications** Hepatocellular carcinoma Sorafenib is indicated for the treatment of patients with unresectable hepatocellular carcinoma (HCC) (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Renal cell carcinoma Sorafenib is indicated for the treatment of patients with advanced renal cell carcinoma who have failed prior systemic therapy or are considered unsuitable for such therapy. Differentiated thyroid carcinoma Sorafenib is indicated for the treatment of patients with locally advanced or metastatic, progressive, differentiated thyroid carcinoma refractory to radioactive iodine treatment.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01EX02
sorafenib
Manufacturer Information
LOTUS INTERNATIONAL PTE. LTD.
Remedica Ltd
PHAROS MT LTD.
Active Ingredients
Documents
Package Inserts
Sorafenib alvogen FCT 200mg PI.pdf
Approved: March 2, 2023
