Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION, CONCENTRATE
**2.2 DOSAGE AND ADMINISTRATION** **General** Substitution by any other biological medicinal product approved in the indication requires the consent of the prescribing physician. _Premedication for infusion-related reactions_ Premedicate with 100 mg IV methylprednisolone (or an equivalent) approximately 30 minutes prior to each Ocrevus infusion (see section _2.4 Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) and with an antihistaminic drug (e.g. diphenhydramine) approximately 30–60 minutes before each infusion of Ocrevus to reduce the frequency and severity of infusion-related reactions. The addition of an antipyretic (e.g. acetaminophen/paracetamol) may also be considered approximately 30–60 minutes before each infusion of Ocrevus. _Administration of Ocrevus_ Ocrevus is administered as an IV infusion through a dedicated line under the close supervision of an experienced healthcare professional with access to appropriate medical support to manage severe reactions such as serious IRRs. Ocrevus infusions should not be administered as an intravenous push or bolus. Use isotonic 0.9% sodium chloride solution as the infusion vehicle. In the event an IV infusion cannot be completed the same day, the remaining liquid in the infusion bag must be discarded (see section _4.1 Storage and 4.2 Special Instructions for Use, Handling and Disposal_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Observe the patient for at least one hour after the completion of the infusion (see section _2.4.1 Warnings and Precautions, General, Infusion-Related Reactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Initial Dose_ Ocrevus is administered by IV infusion as a 600 mg dose every 6 months. The initial 600 mg dose is administered as two separate IV infusions; first as a 300 mg infusion, followed 2 weeks later by a second 300 mg infusion. _Subsequent Doses_ Subsequent doses of Ocrevus thereafter are administered as a single 600 mg IV infusion every 6 months (see Table 1). If patients did not experience a serious infusion-related reaction (IRR) with any previous Ocrevus infusion, a shorter (2-hour) infusion can be administered for subsequent doses (see Table 1, Option 2) (see sections _2.6.1 Undesirable Effects, Clinical Trials_ and _3.1.2 Clinical/Efficacy Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). A minimum interval of 5 months should be maintained between each dose of Ocrevus. 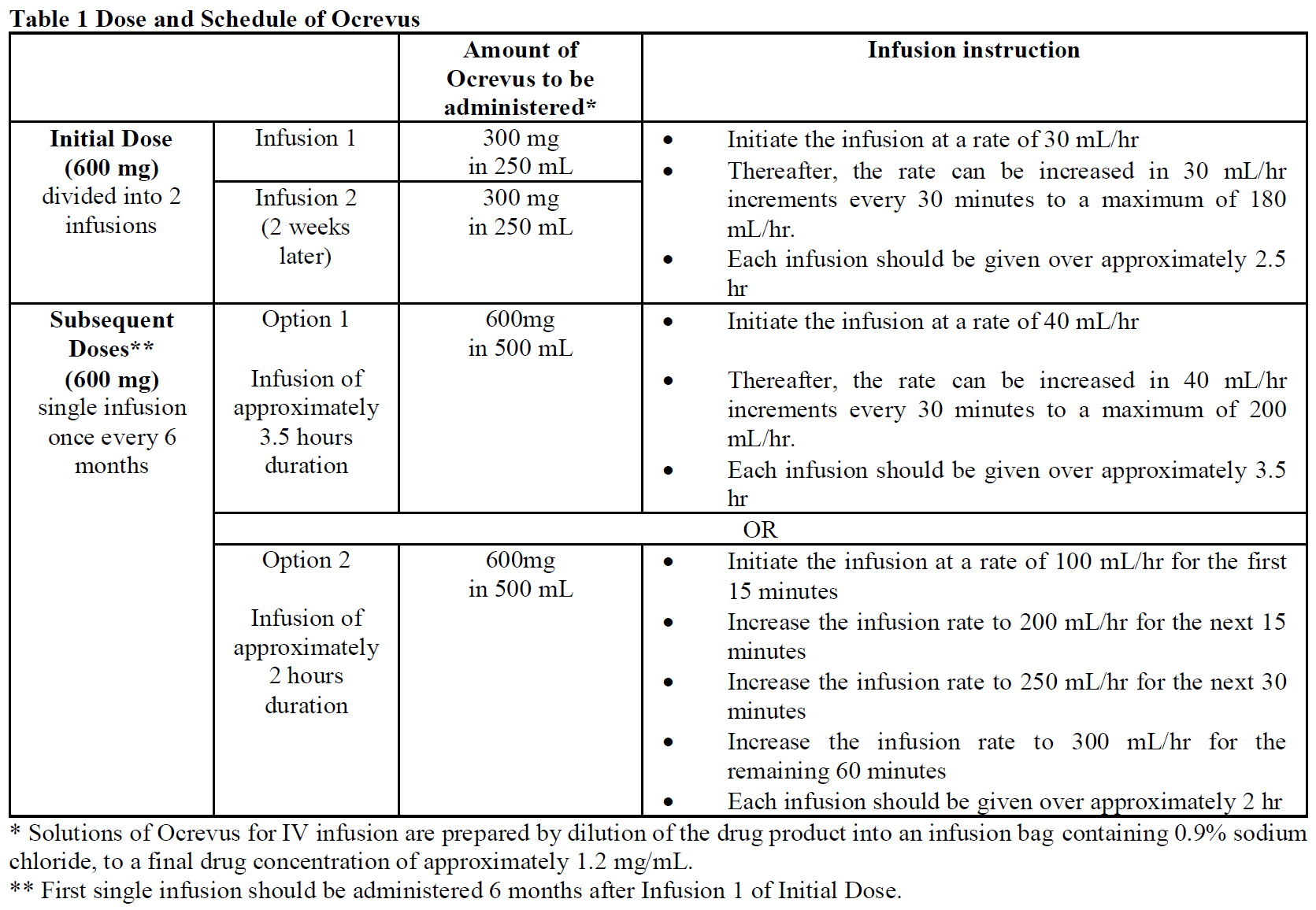 _**Delayed or Missed Doses**_ If a planned infusion of Ocrevus is missed, it should be administered as soon as possible; do not wait until the next planned dose. The treatment interval for Ocrevus should be maintained between doses. _**Infusion Adjustments during Treatment:**_ No dose reductions of Ocrevus are recommended. In case of infusion-related reactions (IRRs) during any infusion, see the following adjustments. Additional information on IRRs can be found in section 2.4.1 Warnings and Precautions, General, Infusion-Related Reactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. _Life-threatening IRRs_ Immediately stop Ocrevus if there are signs of a life-threatening or disabling infusion-related reaction during an infusion, such as acute hypersensitivity or acute respiratory distress syndrome. The patient should receive appropriate supportive treatment. Permanently discontinue Ocrevus in these patients. _Severe IRRs_ If a patient experiences a severe infusion-related reaction or a complex of flushing, fever, and throat pain symptoms, the infusion should be interrupted immediately and the patient should receive symptomatic treatment. The infusion should be restarted only after all symptoms have resolved. The initial infusion rate at restart should be half of the infusion rate at the time of onset of the reaction. _Mild to Moderate IRRs_ If a patient experiences a mild to moderate infusion-related reaction (e.g. headache), the infusion rate should be reduced to half the rate at the onset of the event. This reduced rate should be maintained for at least 30 minutes. If tolerated, the infusion rate may then be increased according to the patient’s initial infusion schedule. See section 2.4.1 Warnings and Precautions, General, Infusion-Related Reactions for full description of symptoms associated with IRRs – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. **2.2.1 Special Dosage Instructions** **Pediatric Use** The safety and efficacy of Ocrevus in children and adolescents (<18 years) has not been studied. **Geriatric Use** The safety and efficacy of Ocrevus in patients ≥55 years of age has not been studied. **Renal Impairment** The safety and efficacy of Ocrevus in patients with renal impairment has not been formally studied. A change in dose is not expected to be required for patients with renal impairment (see section _2.5.6 Use in Special Populations, Renal Impairment and 3.2.5 Pharmacokinetics in Special Populations, Renal Impairment_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic Impairment** The safety and efficacy of Ocrevus in patients with hepatic impairment has not been formally studied. A change in dose is not expected to be required for patients with hepatic impairment (see section _2.5.7 Use in Special Populations, Hepatic Impairment and 3.2.5 Pharmacokinetics in Special Populations, Hepatic Impairment_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS
Medical Information
**2.1 THERAPEUTIC INDICATION(S)** Ocrevus is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis (RMS) with active disease defined by clinical or imaging features, to reduce the frequency of clinical relapses and delay the progression of physical disability. Ocrevus is indicated for the treatment of adult patients with early primary progressive multiple sclerosis (PPMS) with imaging features characteristic of inflammatory activity to delay progression of physical disability.
**2.3 CONTRAINDICATIONS** - Hypersensitivity to ocrelizumab or to any of the excipients. - Severe active infection until resolution (see Section _2.4 Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Patients with severely immunocompromised state (see Section _2.4 Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L04AA36
xl 04 aa 36
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
Roche Diagnostics GmbH
F. Hoffmann-La Roche Ltd
Active Ingredients
Documents
Package Inserts
Ocrevus Approved PI.pdf
Approved: September 28, 2023
