Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**4 Dosage regimen and administration** **Dosage regimen** **General target population** The recommended dose of gallium (68Ga) gozetotide is 1.8 to 2.2 MBq/kg of body weight (0.049 to 0.059 mCi/kg), with a minimum dose of 111 MBq (3 mCi) up to a maximum dose of 259 MBq (7 mCi). **Special populations** **Renal/hepatic impairment** No dose adjustment is required in patients with renal or hepatic impairment (see section 11 Clinical pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Pediatric patients (below 18 years)** The safety and efficacy of gallium (68Ga) gozetotide in pediatric patients below 18 years have not been established. **Geriatric patients (65 years of age or above)** No dose adjustment is required in patients 65 years of age or above (see section 11 Clinical pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Method of administration** After reconstitution, gallium (68Ga) gozetotide solution should be administered by slow intravenous injection, in order to avoid local extravasation resulting in inadvertent radiation exposure to the patient and imaging artifacts. Accidental extravasation may cause local irritation, due to the acidic pH of the gallium (68Ga) gozetotide solution for injection. Cases of extravasation should be managed as per institutional guidelines. The total radioactivity in the syringe should be verified with a dose calibrator immediately before and after gallium (68Ga) gozetotide administration to the patient. The dose calibrator must be calibrated and comply with international standards (see section 14 Pharmaceutical information – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Radiation safety** **Handling** After reconstitution, gallium (68Ga) gozetotide solution for injection should be handled with appropriate safety measures to minimize radiation exposure. Waterproof gloves, effective radiation shielding and other appropriate safety measures should be used when preparing and handling gallium (68Ga) gozetotide solution in order to avoid unnecessary radiation exposure to the occupational workers, clinical personnel, and other persons (see section 14 Pharmaceutical information – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Appropriate aseptic precautions should be taken when withdrawing and administering gallium (68Ga) gozetotide solution for injection (see section 14 Pharmaceutical information – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Radiopharmaceuticals should be used by or under the control of healthcare providers who are qualified by specific training and experience in the safe use and handling of radionuclides. **Patient preparation** Patients should be well hydrated prior to gallium (68Ga) gozetotide administration and should be advised to void immediately prior to and frequently during the first hours after image acquisition to reduce radiation exposure. **Image acquisition** Gallium (68Ga) gozetotide PET image acquisition should be performed by scanning the whole body starting at mid-thigh and proceeding to skull base. PET images should be acquired 50 to 100 minutes after the intravenous administration of gallium (68Ga) gozetotide solution. Imaging acquisition start time and duration should be adapted to the equipment used, the patient and the tumor characteristics, in order to obtain the best image quality possible. **Image interpretation** Gallium (68Ga) gozetotide binds to PSMA on the surface of PSMA-expressing cells. Based on the intensity of the signals, PET images obtained with gallium (68Ga) gozetotide indicate the presence of PSMA protein in tissues. **Radiation dosimetry** The mean effective radiation dose of gallium (68Ga) gozetotide is 0.0166 mSv/MBq, resulting in an approximate effective radiation dose of 4.30 mSv for an administered activity of 259 MBq (7 mCi). Radiation absorbed doses for organs and tissues of adult patients, following intravenous injection of gallium (68Ga) gozetotide are shown in Table 4-1. The highest radiation absorbed dose of gallium (68Ga) gozetotide occurred in the kidneys, salivary glands, bladder wall, lacrimal glands, spleen, and liver. The estimated radiation absorbed doses to these organs for an administered activity of 259 MBq are 64 mGy (kidneys), 25 mGy (salivary glands), 22 mGy (bladder wall), 10 mGy (lacrimal glands), 10 mGy (spleen) and 8 mGy (liver). 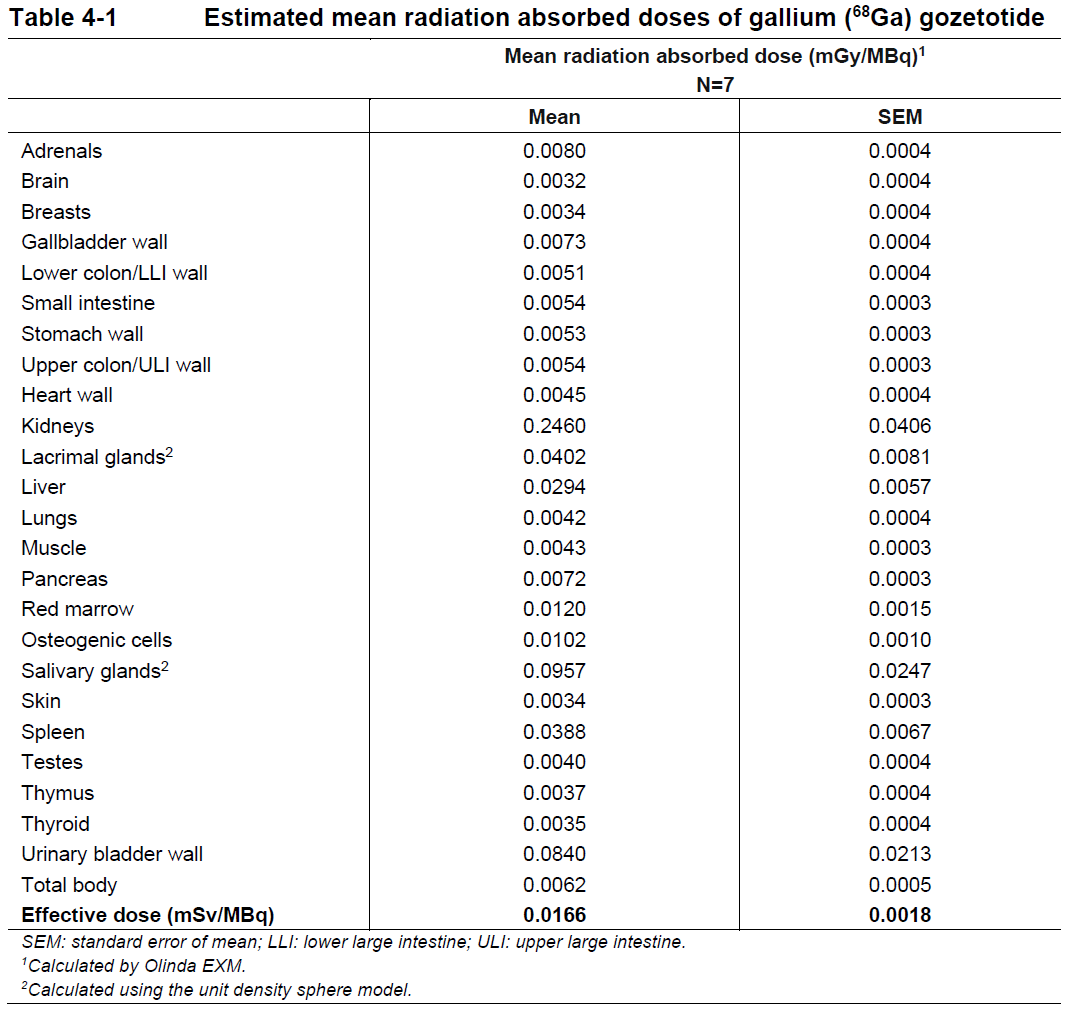 Gallium-68 decays with a half-life of 68 min to stable zinc-68. The principal radiation emission data, radiation attenuation by lead shielding, and physical decay of gallium-68 are shown in Tables 4-2, 4-3 and 4-4. 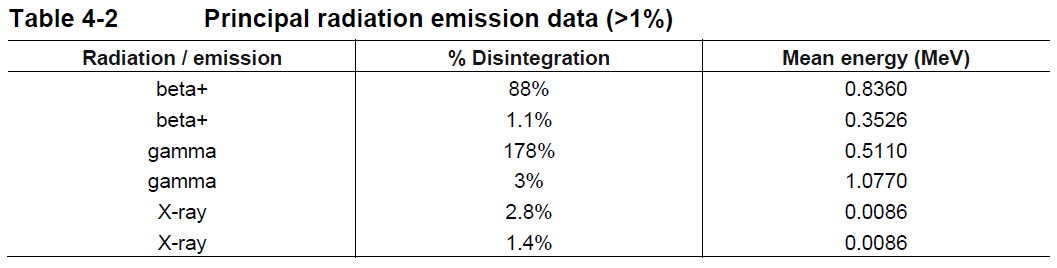 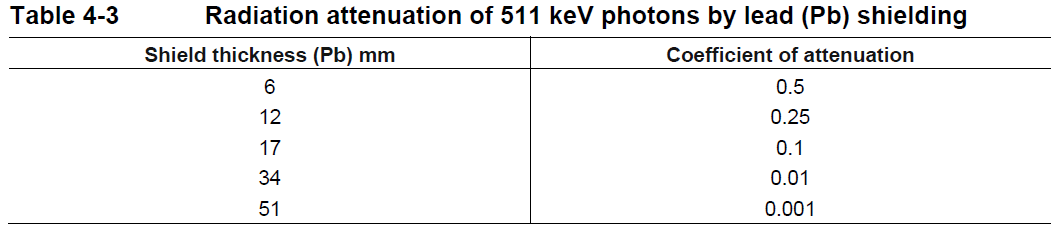 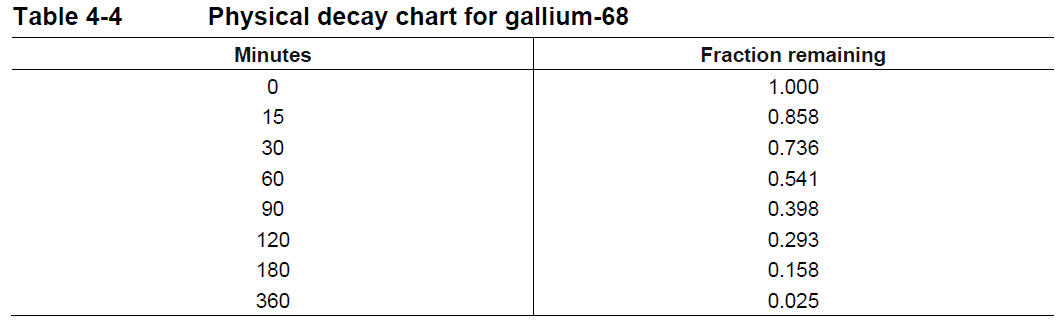
INTRAVENOUS
Medical Information
**3 Indications** Locametz, after radiolabelling with gallium-68, is indicated for the detection of prostate-specific membrane antigen (PSMA)-positive lesions with positron emission tomography (PET) in adults with prostate cancer (PCa) in the following clinical settings: - Primary staging of patients with high-risk PCa prior to primary curative therapy, - Suspected PCa recurrence in patients with increasing levels of serum prostate-specific antigen (PSA) after primary curative therapy, - Identification of patients with PSMA-positive progressive metastatic castration-resistant prostate cancer (mCRPC) for whom PSMA-targeted therapy is indicated.
**5 Contraindications** Hypersensitivity to the active substance, to any of the excipients or to any of the components of the labelled radiopharmaceutical.
V09IX14
gallium (68Ga) gozetotide
Manufacturer Information
NOVARTIS (SINGAPORE) PTE LTD
Advanced Accelerator Applications (Italy) S.r.l.
Active Ingredients
Documents
Package Inserts
Locametz kit PI.pdf
Approved: November 30, 2023
