Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**4.2 Posology and method of administration** This medicinal product should be administered by qualified healthcare professionals and under appropriate medical supervision. Posology The recommended dose is 11 mg/kg siltuximab given over 1 hour as an intravenous infusion administered every 3 weeks until treatment failure. _Treatment criteria_ Haematology laboratory tests should be performed prior to each dose of SYLVANT therapy for the first 12 months and every third dosing cycle thereafter. Before administering the infusion, the prescriber should consider delaying treatment, if the treatment criteria outlined in Table 1 are not met. Dose reduction is not recommended. 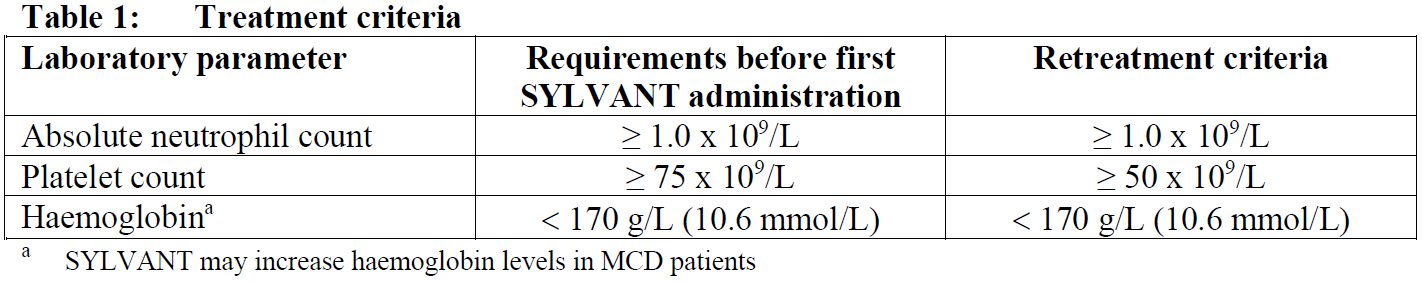 The SYLVANT therapy should be withheld if the patient has a severe infection or any severe non-haematological toxicity and can be restarted at the same dose after recovery. If the patient develops a severe infusion related reaction, anaphylaxis, severe allergic reaction, or cytokine release syndrome related to the infusion, further administration of SYLVANT should be discontinued. Discontinuing the medicinal product should be considered if there are more than 2 dose delays due to toxicities related to the treatment during the first 48 weeks. Special populations _Elderly patients_ No major age-related differences in pharmacokinetics (PK) or in safety profile were observed in clinical studies. No dose adjustment is required (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal and/or hepatic impairment_ No formal studies have been conducted to investigate the PK of siltuximab in patients with renal or hepatic impairment (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric population_ The safety and efficacy of siltuximab in children aged 17 years and younger have not been established. No data are available. Method of administration Siltuximab must be administered as an intravenous infusion. For instructions on reconstitution and dilution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** SYLVANT is indicated for the treatment of adult patients with multicentric Castleman’s disease (MCD) who are human immunodeficiency virus (HIV) negative and human herpesvirus-8 (HHV-8) negative.
**4.3 Contraindications** Severe hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
Pending
xpending
Manufacturer Information
LINK HEALTHCARE SINGAPORE PTE. LTD.
Cilag AG
Active Ingredients
Documents
Package Inserts
Sylvant Powder for Concentrate for Solution for Infusion PI.pdf
Approved: November 5, 2020
