Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** _Restricted to hospital use only._ Spinal anaesthesia must only be administered by (or under the supervision of) specialist medical personnel with the necessary knowledge and experience (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The equipment, drugs and personnel capable of dealing with an emergency, e.g. maintaining the patency of the airways and administering oxygen, must be immediately available, since in rare cases severe reactions, sometimes with a fatal outcome, have been reported after using local anaesthetics, even in the absence of individual hypersensitivity in the patient’s case history. If signs of acute systemic toxicity or total spinal block are observed, the injection of the local anaesthetic must be stopped immediately (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Posology** Posology must be established on an individual basis in accordance with the characteristics of the specific case. When determining the dose, take into consideration the patient’s physical condition and the concomitant administration of other medicinal products. The lowest possible dose should be chosen. The duration of action is dose-dependent. The indications relating to recommended doses are valid in adults of average height and weight (approximately 70 kg) for obtaining an effective block with one single administration. There are wide individual variations with regard to extent and duration of action. The experience of the anaesthetist and knowledge of the patient’s general condition are essential for establishing the dose. With regard to posology the following guidelines are applied: _Adult population_ 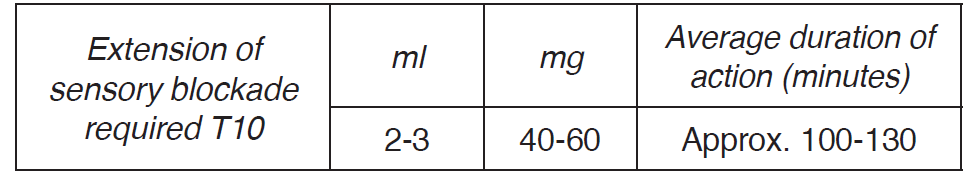 As a general guideline, the maximum recommended dose is 80 mg of prilocaine hydrochloride (= 4 ml Takipril). _Paediatrics population_ The safety and efficacy of Takipril in paediatric population have not been established. No data are available. The use of Takipril in children and adolescents is not recommended. The use of Takipril in children younger than 6 months is contraindicated (see section 4.3). _Special Population_ It is advisable to reduce the dose in patients in a compromised general condition. In addition, in patients with established concomitant disorders (e.g. vascular occlusion, arteriosclerosis, diabetic polyneuropathy) a reduced dose is indicated. In the case of compromised liver or kidney function, a lower dosage range is recommended. **Method of administration** Due to the glucose content, Takipril is only to be used for spinal anaesthesia. It is not recommended for the use in epidural anaesthesia. Inject Takipril via intrathecal route into the intervertebral space L2/L3, L3/L4 and L4/L5. Administer the injection slowly, after having aspirated a minimum quantity of CSF to confirm the correct position and check the patient’s vital functions extremely carefully maintaining continuous verbal contact. If signs of acute systemic toxicity or total spinal block are observed, the injection of the local anaesthetic must be stopped immediately (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If the patient is in a seated position, the injected solution diffuses mainly in a caudal direction (in the direction of the sacrum); if the patient is lying down, the anaesthetic diffuses by gravity according to the patient’s position (Trendelenburg and anti-Trendelenburg). By means of the excipient glucose, the density of Takipril is 1.026 g/g at 20°C, equivalent to 1.021 g/g at 37°C.
INTRATHECAL
Medical Information
**4.1 Therapeutic indications** Takipril is indicated in adults for spinal anaesthesia in short-term surgical procedures (see section 4.2).
**4.3 Contraindications** Takipril must not be used in patients with: - hypersensitivity to prilocaine hydrochloride, other amide-type local anaesthetics or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_, - serious problems with cardiac conduction, - severe anaemia, - decompensated cardiac insufficiency, - cardiogenic and hypovolemic shock, - congenital or acquired methemoglobinemia, - concomitant anticoagulant therapy, - general and specific contraindications to spinal anaesthesia regardless of the local anaesthetic used should be taken into account. The use of Takipril in children younger than 6 months is contraindicated due to a higher risk of developing methemoglobinemia. The intravascular injection of Takipril is contraindicated. Takipril must not be injected into infected areas.
N01BB04
prilocaine
Manufacturer Information
B. Braun Singapore Pte Ltd.
Sintetica SA
Active Ingredients
Documents
Package Inserts
Takipril Solution for Injection PI.pdf
Approved: September 17, 2021
