Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**4.2. Posology and method of administration** _ReliTrexed_ ® must only be administered under the supervision of a physician qualified in the use of anti-cancer chemotherapy. _ReliTrexed_ ® in combination with cisplatin: The recommended dose of _ReliTrexed_ ® is 500 mg/m2 of body surface area (BSA) administered as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. The recommended dose of cisplatin is 75 mg/m2 BSA infused over two hours approximately 30 minutes after completion of the _ReliTrexed_ ® infusion on the first day of each 21-day cycle. Patients must receive adequate anti-emetic treatment and appropriate hydration prior to and/or after receiving cisplatin (see cisplatin package insert for specific dosing advice). _ReliTrexed_ ® in combination with pembrolizumab and platinum chemotherapy: The recommended dose of _ReliTrexed_ ® when administered with pembrolizumab and platinum chemotherapy for the initial treatment of metastatic non-squamous NSCLC in patients with a creatinine clearance (calculated by Cockcroft-GauIt equation) of 45 mL/min or greater is 500 mg/m2 as an intravenous infusion over 10 minutes administered after pembrolizumab and prior to carboplatin or cisplatin on Day I of each 21-day cycle for 4 cycles. Following completion of platinum-based therapy, treatment with _ReliTrexed_ ® with or without pembrolizumab is administered until disease progression or unacceptable toxicity. Please refer to the full prescribing information for pembrolizumab and for carboplatin or cisplatin. _ReliTrexed_ ® as single agent: In patients treated for NSCLC after prior chemotherapy, the recommended dose of _ReliTrexed_ ® is 500 mg/m2 BSA administered as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. Pre-medication Regimen: To reduce the incidence and severity of skin reactions, a corticosteroid should be given the day prior to, on the day of, and the day after _ReliTrexed_ ® administration. The corticosteroid should be equivalent to 4 mg of dexamethasone administered orally twice a day (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). To reduce toxicity, patients treated with _ReliTrexed_ ® must also receive vitamin supplementation (see section 4 4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients must take oral folic acid or a multivitamin containing folic acid (350–1000mcg) on a daily basis. The most commonly used dose of oral folic acid in clinical trials was 400mcg. At least five doses of folic acid must be taken during the seven days preceding the first dose of _ReliTrexed_ ® and dosing must continue during the full course of therapy and for 21 days after the last dose of _ReliTrexed_ ®. Patients must also receive an intramuscular injection of vitamin B12 (1000mcg) in the week preceding the first dose of _ReliTrexed_ ® and once every three cycles thereafter. Subsequent vitamin B12 injections may be given on the same day as _ReliTrexed_ ®. Monitoring: Patients receiving _ReliTrexed_ ® should be monitored before each dose with a complete blood count, including a differential white cell count (WCC) and platelet count. Prior to each chemotherapy administration blood chemistry tests should be collected to evaluate renal and hepatic function. Before the start of any cycle of chemotherapy, patients are required to have the following: Absolute Neutrophil Count (ANC) should be 1500 cells/mm3 and platelets should be 100,000 cells/mm3. Creatinine clearance should be ≥ 45 ml/min. The total bilirubin should be ≤ 1.5 times upper limit of normal. Alkaline phosphatase (AP), aspartate aminotransferase (AST or SGOT) and alanine aminotransferase (ALT or SGPT) should be ≤ 3 times upper limit of normal. Alkaline phosphatase, AST and ALT ≤ 5 times upper limit of normal is acceptable if liver has tumour involvement. Dose adjustments Dose adjustments at the start of a subsequent cycle should be based on nadir haematologic counts or maximum non-haematologic toxicity from the preceding cycle of therapy. Treatment may be delayed to allow sufficient time for recovery. Upon recovery patients should be retreated using the guidelines in Tables 1, 2 and 3, which are applicable for _ReliTrexed_ ® used as a single agent or in combination with cisplatin. 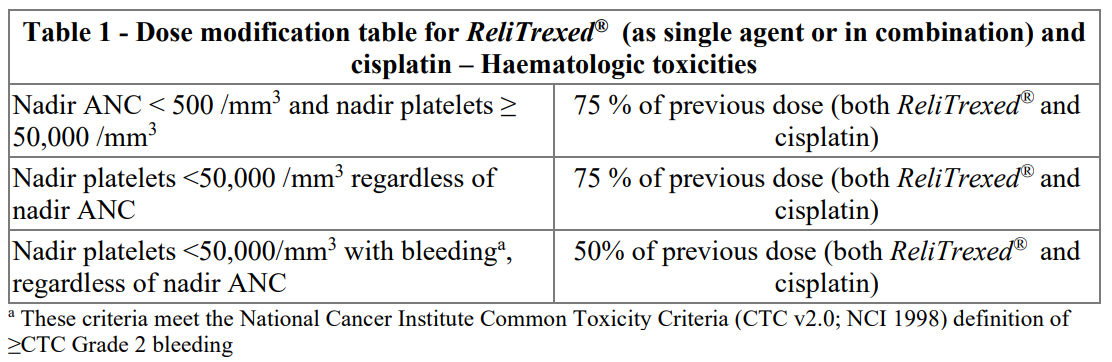 If patients develop non-haematologic toxicities ≥ Grade 3 (excluding neurotoxicity), _ReliTrexed_ ® should be withheld until resolution to less than or equal to the patient's pre-therapy value. Treatment should be resumed according to the guidelines in Table 2. 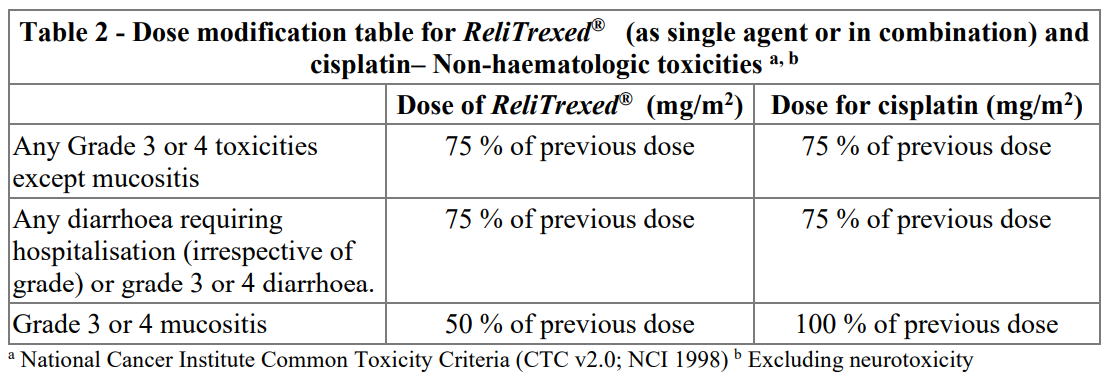 In the event of neurotoxicity, the recommended dose adjustment for _ReliTrexed_ ® and cisplatin is documented in Table 3. Patients should discontinue therapy if Grade 3 or 4 neurotoxicity is observed. 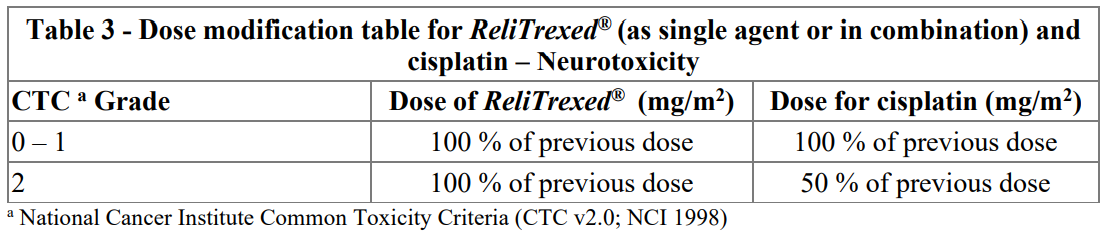 Treatment with _ReliTrexed_ ® should be discontinued if a patient experiences any haematologic or non-haematologic Grade 3 or 4 toxicity after 2 dose reductions or immediately if Grade 3 or 4 neurotoxicity is observed. _Special populations_ _Elderly_ In clinical studies, there has been no indication that patients 65 years of age or older are at increased risk of adverse reaction compared to patients younger than 65 years old. No dose reductions other than those recommended for all patients are necessary. _Paediatric population_ _ReliTrexed_ ® is not recommended for use in children below 18 years of age, due to insufficient data on safety and efficacy. _Patients with renal impairment (standard cockcroft and gault formula or glomerular filtration rate measured Tc99m-DPTA serum clearance method)_ Pemetrexed is primarily eliminated unchanged by renal excretion. In clinical studies, patients with creatinine clearance of ≥ 45 ml/min required no dose adjustments other than those recommended for all patients. There are insufficient data on the use of pemetrexed in patients with creatinine clearance below 45 ml/min; therefore the use of pemetrexed is not recommended (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Patients with hepatic impairment_ No relationships between AST (SGOT), ALT (SGPT), or total bilirubin and pemetrexed pharmacokinetics were identified. However patients with hepatic impairment such as bilirubin > 1.5 times the upper limit of normal and/or aminotransferase > 3.0 times the upper limit of normal (hepatic metastases absent) or > 5.0 times the upper limit of normal (hepatic metastases present) have not been specifically studied. Method of administration _ReliTrexed_ ® is for intravenous use. _ReliTrexed_ ® should be administered as an intravenous infusion over 10 minutes on the first day of each 21-day cycle. For precautions to be taken before handling or administering _ReliTrexed_ ® and for instructions on reconstitution and dilution of _ReliTrexed_ ® before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** Malignant pleural mesothelioma (MPM) _ReliTrexed_ ® in combination with cisplatin is indicated for the treatment of chemotherapy naïve patients with unresectable malignant pleural mesothelioma or who otherwise not candidates for curative surgery. Non-small cell lung cancer (NSCLC) (see sections 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_): _ReliTrexed_ ® in combination with cisplatin is indicated for the first line treatment of patients with locally advanced or metastatic non-squamous NSCLC. _ReliTrexed_ ® in combination with pembrolizumab and platinum chemotherapy, is indicated for the first-line treatment of patients with metastatic non-squamous NSCLC, with no EGFR or ALK genomic tumor aberrations. _ReliTrexed_ ® is indicated as monotherapy for the maintenance treatment of locally advance or metastatic non-squamous NSCLC in patients whose disease has not progressed immediately following first-line treatment with platinum-based chemotherapy. _ReliTrexed_ ® is indicated as monotherapy for the second line treatment of patients with locally advanced or metastatic non-squamous NSCLC.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Breast-feeding (see section 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Concomitant yellow fever vaccine (see section 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01BA04
pemetrexed
Manufacturer Information
WELLCHEM PHARMACEUTICALS PTE LTD
Reliance Life Sciences Pvt. Ltd., ( Plant 6)
Active Ingredients
Documents
Package Inserts
PI_ 18 May.pdf
Approved: June 24, 2022
