Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2 DOSAGE AND ADMINISTRATION** **2.1 Recommended Dosage** **Compensated Liver Disease** The recommended dose of BARACLUDE for chronic hepatitis B virus infection in nucleoside-treatment-naïve adults and adolescents 16 years of age and older is 0.5 mg once daily, with or without food. The recommended dose of BARACLUDE in adults and adolescents (≥16 years of age) with a history of hepatitis B viremia while receiving lamivudine or known lamivudine resistance mutations is 1 mg once daily, which must be taken on an empty stomach (at least 2 hours after a meal and 2 hours before the next meal). _**Decompensated Liver Disease**_ The recommended dose of BARACLUDE for chronic hepatitis B virus infection in adults with decompensated liver disease is 1 mg once daily, which must be taken on an empty stomach (at least 2 hours after a meal and 2 hours before the next meal). **Oral Solution** BARACLUDE (entecavir) Oral Solution contains 0.05 mg of entecavir per milliliter. Therefore, 10 mL of the oral solution provides a 0.5 mg dose and 20 mL provides a 1 mg dose of entecavir. **2.2 Renal Impairment** In subjects with renal impairment, the apparent oral clearance of entecavir decreased as creatinine clearance decreased \[see _Clinical Pharmacology (10.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. Dosage adjustment is recommended for patients with creatinine clearance less than 50 mL/min, including patients on hemodialysis or continuous ambulatory peritoneal dialysis (CAPD), as shown in Table 1. The once-daily dosing regimens are preferred. 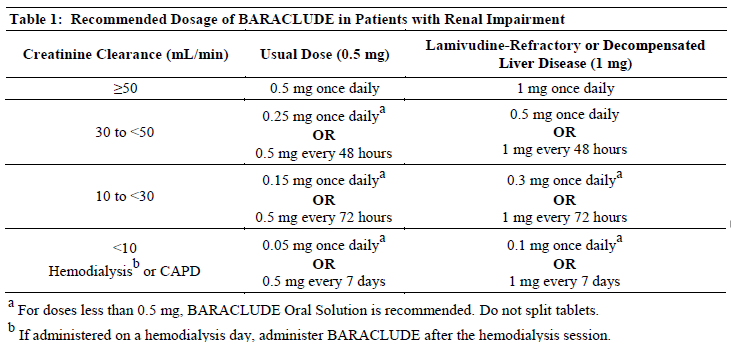 **2.3 Hepatic Impairment** No dosage adjustment is necessary for patients with hepatic impairment. **2.4 Duration of Therapy** The optimal duration of treatment with BARACLUDE for patients with chronic hepatitis B virus infection and the relationship between treatment and long-term outcomes such as cirrhosis and hepatocellular carcinoma are unknown.
ORAL
Medical Information
**1 INDICATIONS AND USAGE** BARACLUDE® (entecavir) is indicated for the treatment of chronic hepatitis B virus infection in adults with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease. The following points should be considered when initiating therapy with BARACLUDE: - This indication is based on histologic, virologic, biochemical, and serologic responses in nucleoside-treatment-naïve and lamivudine-resistant adult subjects with HBeAg-positive or HBeAg-negative chronic HBV infection with compensated liver disease \[see _Clinical Studies (12)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. - Virologic, biochemical, serologic, and safety data are available from a controlled study in adult subjects with chronic HBV infection and decompensated liver disease \[see _Adverse Reactions (5)_ and _Clinical Studies (12.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. - Virologic, biochemical, serologic, and safety data are available for a limited number of adult subjects with HIV/HBV co-infection who have received prior lamivudine therapy \[see _Warnings and Precautions (4.2)_ and _Clinical Studies (12.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\].
**3 CONTRAINDICATIONS** BARACLUDE is contraindicated in patients with previously demonstrated hypersensitivity to entecavir or any component of the product.
J05AF10
entecavir
Manufacturer Information
BRISTOL-MYERS SQUIBB (SINGAPORE) PTE. LTD.
AstraZeneca Pharmaceuticals LP
CATALENT ANAGNI S.R.L. (Primary and secondary packaging)
Patheon INC.
Active Ingredients
Documents
Package Inserts
Baraclude PI.pdf
Approved: July 22, 2020
