Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**4.2 Posology and method of administration** Treatment with Inrebic should be initiated and monitored under the supervision of physicians experienced in the use of anti-cancer medicinal products. Posology Patients who are on treatment with ruxolitinib, prior to starting treatment with Inrebic, must taper and discontinue ruxolitinib according to the ruxolitinib prescribing information. Baseline testing of thiamine (vitamin B1) levels, complete blood count, hepatic panel, amylase/lipase, blood urea nitrogen (BUN) and creatinine should be obtained prior to starting treatment with Inrebic, periodically during treatment and as clinically indicated. Inrebic treatment should not be started in patients with thiamine deficiency, until thiamine levels have been corrected (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Initiating treatment with Inrebic is not recommended in patients with a baseline platelet count below 50 x 109/L and ANC < 1.0 x 109/L. It is recommended that prophylactic anti-emetics be used according to local practice for the first 8 weeks of treatment and continued thereafter as clinically indicated (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Administration of Inrebic with a high fat meal may reduce the incidence of nausea and vomiting. The recommended dose of Inrebic is 400 mg once daily. Treatment may be continued for as long as patients derive clinical benefit. Dose modifications should be considered for haematologic and non-haematologic toxicities (Table 1). Inrebic should be discontinued in patients who are unable to tolerate a dose of 200 mg daily. If a dose is missed, the next scheduled dose should be taken the following day. Extra capsules should not be taken to make up for the missed dose. _Dose modifications_ Dose modifications for haematologic toxicities, non-haematologic toxicities and management of Wernicke’s encephalopathy (WE) are shown in Table 1. _Dose management of thiamine levels_ Before treatment initiation and during treatment, thiamine levels should be replenished if they are low. During treatment, thiamine levels should be assessed periodically (e.g. monthly for the first 3 months and every 3 months thereafter) and as clinically indicated (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Dose modifications with concomitant use of strong CYP3A4 inhibitors_ If concomitant strong CYP3A4 inhibitors cannot be avoided, the dose of Inrebic should be reduced to 200 mg. Patients should be carefully monitored (e.g. at least weekly) for safety (see section 4.4 and 4.5 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In cases where co-administration with a strong CYP3A4 inhibitor is discontinued, the Inrebic dose should be increased to 300 mg once daily during the first two weeks after discontinuation of the CYP3A4 inhibitor and then 400 mg once daily thereafter as tolerated. Additional dose adjustments should be made as needed, based upon monitoring of Inrebic-related safety and efficacy. _Dose re-escalation_ If the adverse reaction due to Inrebic that resulted in a dose reduction is controlled with effective management and the toxicity is resolved for at least 28 days, the dose level may be re-escalated to one dose level higher per month up to the original dose level. Dose re-escalation is not recommended if the dose reduction was due to a Grade 4 non-haematologic toxicity, ≥ Grade 3 alanine aminotransferase (ALT), aspartate aminotransferase (AST), or total bilirubin elevation, or reoccurrence of a Grade 4 haematologic toxicity. 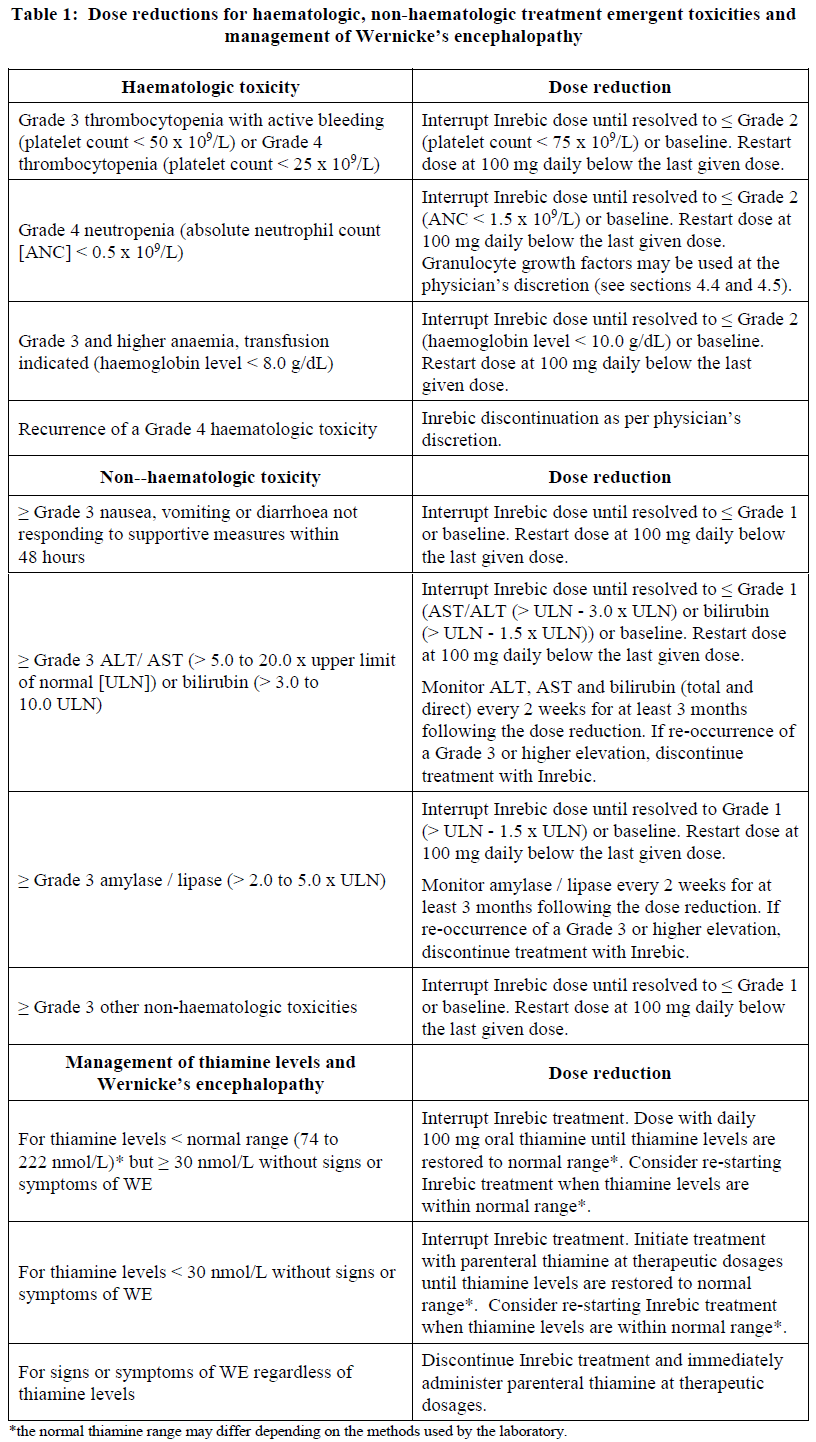 _Special populations_ _Renal impairment_ For patients with severe renal impairment (creatinine clearance \[CLcr\] 15 mL/min to 29 mL/min by Cockcroft-Gault \[C-G\]), the dose should be reduced to 200 mg. No modification of the starting dose is recommended for patients with mild to moderate renal impairment (CLcr 30 mL/min to 89 mL/min by C-G). Due to potential increase of exposure, patients with pre-existing moderate renal impairment may require at least weekly safety monitoring and if necessary, dose modifications based on adverse reactions. _Hepatic impairment_ Inrebic pharmacokinetics have not been evaluated in patients with severe hepatic impairment. Use of Inrebic in patients with severe hepatic impairment (Child-Pugh class C or total bilirubin >3 times ULN and any AST increase) should be avoided. No modification of the starting dose is required for patients with mild to moderate hepatic impairment. _Elderly_ No additional dose adjustments are required in elderly patients (> 65 years of age). _Paediatric population_ The safety and efficacy of Inrebic in children and adolescents aged up to 18 years have not been established. No data are available. Method of administration Inrebic is for oral use. The capsules should not be opened, broken or chewed. They should be swallowed whole, preferably with water, and may be taken with or without food. Administration with a high fat meal may reduce the incidence of nausea and vomiting, therefore it is recommended to be taken with food.
ORAL
Medical Information
**4.1 Therapeutic indications** Inrebic is indicated for the treatment of splenomegaly and/or disease related symptoms in adult patients with intermediate-2 or high-risk primary myelofibrosis, post-polycythemia vera myelofibrosis or post-essential thrombocythemia myelofibrosis, including patients who have been previously exposed to ruxolitinib.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Pregnancy (see section 4.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01EJ02
fedratinib
Manufacturer Information
BRISTOL-MYERS SQUIBB (SINGAPORE) PTE. LTD.
Celgene International Sarl
Active Ingredients
Documents
Package Inserts
Inrebic Capsules 100mg PI.pdf
Approved: September 29, 2022
