Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**4.2. Posology and method of administration** **TRANEXAMIC ACID MUST NOT BE USED FOR INTRATHECAL OR EPIDURAL ADMINISTRATION.** The recommended standard dose is 5 mL to 10 mL by slow intravenous injection at a rate of 1 mL/minute or 2 to 3 tablets of 0.5 g 2 to 3 times daily. For the indications listed below, the following doses are recommended: _General fibrinolysis_: 1.0 g (2 ampoules of 5 mL) by slow intravenous injection every 6 to 8 hours. _Prostatectomy_: 0.5 g to 1.0 g (1 to 2 ampoules of 5 mL) by slow intravenous injection every 6 to 8 hours (the first injection being given during the operation) for the first 3 days after surgery, thereafter 1 g to 1.5 g orally (2 to 3 tablets) 2 to 3 times daily until macroscopic haematuria is no longer present. _Haematuria_: 1 g to 1.5 g orally (2 to 3 tablets) 2 to 3 times daily until macroscopic haematuria is no longer present. _Epistaxis_: 1.5 g orally (3 tablets) 3 times a day should be administered for 4 to 10 days. Cyklokapron solution for injection may be applied topically to the nasal mucosa of patients suffering from epistaxis. This can be done by soaking a gauze strip in the solution, and then packing the nasal cavity. _Menorrhagia_: 1 g to 1.5 g orally (2 to 3 tablets) 3 to 4 times daily for 3 to 4 days. Cyklokapron therapy is initiated when bleeding has become profuse. _Conisation of the cervix_: 1.5 g orally (3 tablets) 3 times a day for 12 to 14 days postoperatively. _Dental extraction in patients with coagulopathies_: Immediately before surgery, Cyklokapron 10 mg/kg body weight should be given intravenously. After surgery, 25 mg/kg body weight is given orally 3 to 4 times daily for 6 to 8 days. Coagulation factor concentrate might be necessary to administrate. This decision should be taken after consulting specialists on coagulation. _Hereditary angioneurotic oedema_: 1 g to 1.5 g orally (2 to 3 tablets) 2 to 3 times daily as intermittent or continuous treatment, depending on whether the patient has prodromal symptoms or not. For patients with moderate to severe impaired renal function, the following dosages are recommended: 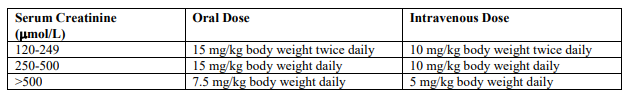
INTRAVENOUS
Medical Information
**4.1. Therapeutic indications** - Haemorrhage or risk of haemorrhage in increased fibrinolysis or fibrinogenolysis. Local fibrinolysis may occur in the following conditions: Menorrhagia, prostatectomy and bladder surgery, haematuria, gastrointestinal haemorrhage, epistaxis, ulcerative colitis, conisation of the cervix, and dental extraction in patients with coagulopathies. General fibrinolysis may occur in the following conditions and situations: Prostatic and pancreatic cancer, after thoracic and other major surgery, in obstetrical complications such as ablatio placentae and postpartum haemorrhage, leukemia, liver diseases, in connection with thrombolytic therapy with streptokinase. - Hereditary angioneurotic oedema.
**4.3. Contraindications** Intrathecal and epidural administration of tranexamic acid is contraindicated. Active thromboembolic disease, such as deep vein thrombosis, pulmonary embolism and cerebral thrombosis. Subarachnoid haemorrhage. The limited clinical experience shows that a reduced risk for re-bleeding is offset by an increase in the rate of cerebral ischaemia. Hypersensitivity to tranexamic acid or any of the ingredients.
B02AA02
tranexamic acid
Manufacturer Information
PFIZER PRIVATE LIMITED
PFIZER MANUFACTURING BELGIUM NV
Active Ingredients
Documents
Package Inserts
Cyklokapron PI.pdf
Approved: August 11, 2021
