Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and Method of Administration** **ALK Testing** A validated ALK assay is necessary for the selection of ALK-positive NSCLC patients. ALK-positive NSCLC status should be established prior to initiation of ALUNBRIG® therapy. **Dosage** The recommended starting dose of ALUNBRIG® is 90 mg once daily for the first 7 days, then 180 mg once daily. Treatment should continue as long as clinical benefit is observed. If a dose of ALUNBRIG® is missed or vomiting occurs after taking a dose, an additional dose should not be administered and the next dose of ALUNBRIG® should be taken at the scheduled time. _**Dose Adjustments**_ Dosing interruption and/or dose reduction may be required based on individual safety and tolerability. ALUNBRIG® dose reduction levels are summarised in Table 1. 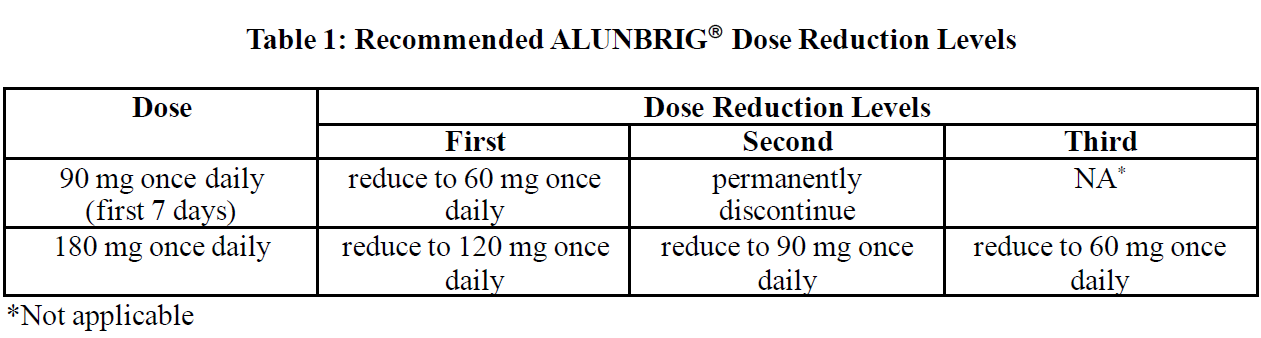 Permanently discontinue ALUNBRIG® if patient is unable to tolerate the 60 mg once daily dose. If ALUNBRIG® is interrupted for 14 days or longer for reasons other than adverse reactions, treatment should be resumed at 90 mg once daily for 7 days before increasing to the previously tolerated dose. Recommendations for dose modifications of ALUNBRIG® for the management of adverse reactions are summarized in Table 2. 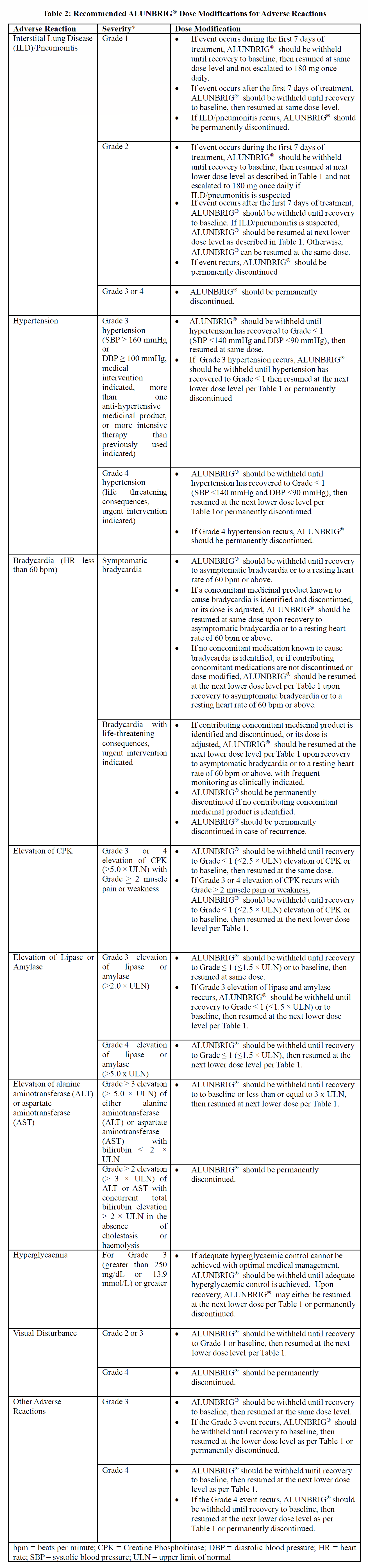 _**Special Patient Populations**_ **Elderly Patients** The limited data on the safety and efficacy of ALUNBRIG® in patients aged 65 years and older suggest that a dose adjustment is not required in elderly patients (see ACTION AND CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). There are limited data on patients over 85 years of age. **Pediatric Patients** The safety and efficacy of ALUNBRIG® in patients less than 18 years of age have not been established. No data are available. **Impaired Renal Function** No dose adjustment is recommended for patients with mild or moderate renal impairment (creatinine clearance ≥ 30 mL/min). The dose of Alunbrig should be reduced by approximately 50% (e.g., from 180 mg to 90 mg, or from 90 mg to 60 mg) for patients with severe renal impairment (eGFR < 30 mL/min) (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients with severe renal impairment should be closely monitored for new or worsening respiratory symptoms that may indicate ILD/pneumonitis (e.g., dyspnoea, cough, etc.) particularly in the first week. (see ACTION AND CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Impaired Hepatic Function** No dose adjustment of Alunbrig is required for patients with mild hepatic impairment (Child-Pugh class A) or moderate hepatic impairment (Child-Pugh class B). The dose of Alunbrig should be reduced by approximately 40% (e.g., from 180 mg to 120 mg, 120 mg to 90 mg, or from 90 mg to 60 mg) for patients with severe hepatic impairment (Child-Pugh class C) . (see ACTION AND CLINICAL PHARMACOLOGY – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Method of Administration** ALUNBRIG® is for oral use. The tablets should be swallowed whole and with water. Do not crush or chew tablets. ALUNBRIG® may be taken with or without food.
ORAL
Medical Information
**4.1 Therapeutic Indications** ALUNBRIG® is indicated for the treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC) previously not treated with an ALK inhibitor. ALUNBRIG® is indicated for the treatment of adult patients with ALK-positive advanced NSCLC previously treated with crizotinib.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01XE43
xl 01 xe 43
Manufacturer Information
TAKEDA PHARMACEUTICALS (ASIA PACIFIC) PTE. LTD.
PENN PHARMACEUTICAL SERVICES LIMITED
Takeda Ireland Limited
Active Ingredients
Documents
Package Inserts
Alunbrig PI.pdf
Approved: October 8, 2021
