Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**Posology and Method of Administration** The use of JEVTANA should be confined to units specialised in the administration of cytotoxics and it should only be administered under the supervision of a physician experienced in the use of anticancer chemotherapy. Facilities and equipment for the treatment of serious hypersensitivity reactions like hypotension and bronchospasm must be available (see section _Special Warnings and Precautions for Use_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Premedication The recommended premedication regimen should be performed at least 30 minutes prior to each administration of JEVTANA with the following intravenous medicinal products to mitigate the risk and severity of hypersensitivity - antihistamine (dexchlorpheniramine 5 mg or diphenhydramine 25 mg or equivalent), - corticosteroid (dexamethasone 8 mg or equivalent), and - H2 antagonist (ranitidine or equivalent) (see section _Special Warnings and Precautions for Use_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Antiemetic prophylaxis is recommended and can be given orally or intravenously as needed. Throughout the treatment, adequate hydration of the patient needs to be ensured, in order to prevent complications like renal failure. Posology The recommended dose of JEVTANA is 25 mg/m2 administered as a 1 hour intravenous infusion every 3 weeks in combination with oral prednisone or prednisolone 10 mg administered daily throughout treatment. _Dose Adjustments_ Dose modifications should be made if patients experience the following adverse reactions (Grades refer to Common Terminology Criteria for Adverse Events (CTCAE 4.0)): 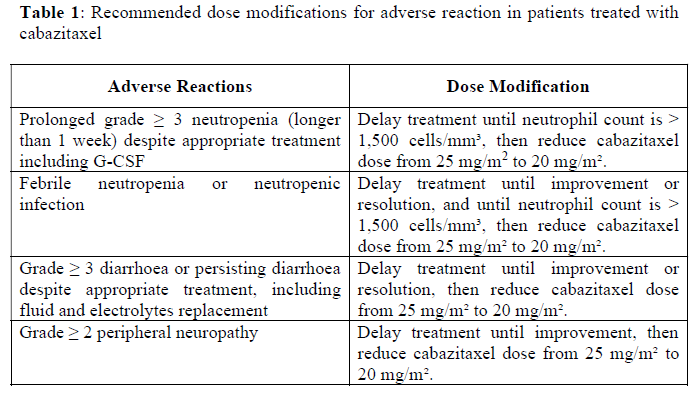 If patients continue to experience any of these reactions at 20 mg/m2, further dose reduction to 15mg/m2 or discontinuation of JEVTANA may be considered. Data in patients below the 20 mg/m2 dose are limited. Special Populations _Patients with Hepatic Impairment_ Cabazitaxel is extensively metabolised by the liver. Patients with mild hepatic impairment (total bilirubin >1 to ≤1.5 x Upper Limit of Normal (ULN) or Aspartate Aminotransferase (AST) >1.5 x ULN), should have cabazitaxel dose reduced to 20 mg/m2. Administration of cabazitaxel to patients with mild hepatic impairment should be undertaken with caution and close monitoring of safety. In patients with moderate hepatic impairment (total bilirubin >1.5 to ≤ 3.0 x ULN), the maximum tolerated dose (MTD) was 15 mg/m2. If the treatment is envisaged in patients with moderate hepatic impairment the dose of cabazitaxel should not exceed 15 mg/m2. However, limited efficacy data are available at this dose. Cabazitaxel should not be given to patients with severe hepatic impairment (total bilirubin >3 x ULN) (see sections _Contraindications_, _Special Warnings and Precautions for Use_ and _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Patients with Renal Impairment_ Cabazitaxel is minimally excreted through the kidney. No dose adjustment is necessary in patients with renal impairment, not requiring hemodialysis. Patients presenting end-stage renal disease (creatinine clearance (CLCR< 15 mL/min/1.73 m2), by their condition and the limited amount of data available should be treated with caution and monitored carefully during treatment (see sections _Special Warnings and Precautions for Use_ and _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Elderly_ No specific dose adjustment for the use of cabazitaxel in elderly patients is recommended (see also sections _Special Warnings and Precautions for Use_, _Undesirable Effects_ and _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Concomitant Medicinal Products Use_ Concomitant medicinal products that are strong inducers or strong inhibitors of CYP3A should be avoided. However, if patients require co-administration of a strong CYP3A inhibitor, a 25% cabazitaxel dose reduction should be considered (see sections _Special Warnings and Precautions for Use_ and _Interaction with Other Medicinal Products and Other Forms of Interaction_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Paediatric Population_ There is no relevant use of JEVTANA in the paediatric population. The safety and the efficacy of JEVTANA in children and adolescents below 18 years of age have not been established. Method of Administration JEVTANA is for intravenous use. For instructions on preparation and administration of the medicinal product, see section _Special Precautions for Disposal and Other Handling_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. PVC infusion containers and polyurethane infusion sets should not be used. JEVTANA must not be mixed with any other medicinal products than those mentioned in section _Special Precautions for Disposal and Other Handling_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**Therapeutic Indications** JEVTANA in combination with prednisone or prednisolone is indicated for the treatment of adult patients with metastatic castration resistant prostate cancer previously treated with a docetaxel-containing regimen.
**Contraindications** - Hypersensitivity to cabazitaxel, to other taxanes, to polysorbate 80 or to any of the excipients listed in _List of Excipients_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. - Neutrophil counts less than 1,500/mm3. - Severe hepatic impairment (total bilirubin >3 x ULN). - Concomitant vaccination with yellow fever vaccine (see section _Interaction with Other Medicinal Products and Other Forms of Interaction_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01CD04
cabazitaxel
Manufacturer Information
SANOFI-AVENTIS SINGAPORE PTE. LTD.
Sanofi-Aventis Deutschland GmbH (Drug Product & Solvent)
Active Ingredients
Documents
Package Inserts
Jevtana concentrate and solvent for solution PI.pdf
Approved: October 25, 2018
