Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, CHEWABLE
**VI. DOSAGE AND ADMINISTRATION** Raltegravir is available as a 400 mg tablet formulation and as a chewable tablet formulation in 100 mg (scored) and 25 mg strengths. The maximum dose of the chewable tablet is 300 mg twice daily. Because the formulations are not bioequivalent, do not substitute chewable tablets for the 400 mg tablet. Raltegravir can be administered with or without food (see VII. Clinical Pharmacology – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Raltegravir is to be given in a combination regimen with other antiretroviral agents. For the treatment of patients with HIV-1 infection, the dosage of raltegravir is as follows: **Adults:** One 400 mg tablet administered orally twice daily **Children and adolescents:** - If at least 25 kg: One 400 mg tablet administered orally twice daily - If unable to swallow a tablet, consider the chewable tablet, as specified in Table 1 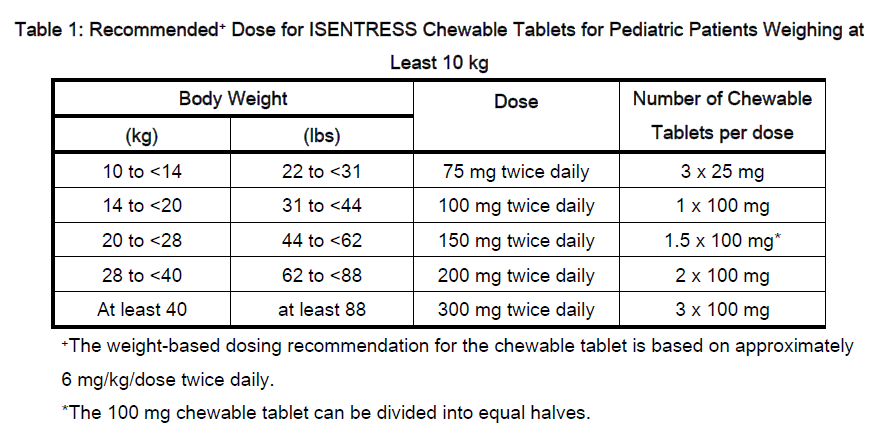
ORAL
Medical Information
**V. INDICATIONS** **Adults** Raltegravir is indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection in adult patients. This indication is based on analyses of plasma HIV-1 RNA levels in three double-blind controlled studies of raltegravir. Two of these studies were conducted in clinically advanced, 3-class antiretroviral (NNRTI, NRTI, PI) treatment-experienced adults through 96 weeks and one was conducted in treatment-naïve adults through 240 weeks. The use of other active agents with raltegravir is associated with a greater likelihood of treatment response (see VIId. Clinical Studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Pediatrics** Raltegravir is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in children and adolescents 2 years of age and older. This indication is based on the evaluation of safety, tolerability, pharmacokinetic parameters and efficacy of raltegravir through at least 24-weeks in a multi-center, open-label, noncomparative study in HIV-1 infected, treatment-experienced children and adolescents 2 to 18 years of age (see VIId. Clinical Studies – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The safety and efficacy of raltegravir 400 mg tablets have not been established in children less than 6 years of age. The safety and efficacy of raltegravir chewable tablets have not been established in children less than 2 years of age.
**VIII. CONTRAINDICATIONS** ISENTRESS-G Film-Coated Tablets and ISENTRESS Chewable Tablets are contraindicated in patients who are hypersensitive to any component of this medicine.
J05AX08
xj 05 ax 08
Manufacturer Information
MSD PHARMA (SINGAPORE) PTE. LTD.
Patheon Pharmaceuticals Inc.
Merck Sharp & Dohme BV
Active Ingredients
Documents
Package Inserts
Isentress Tablet (Grey)_PI.pdf
Approved: April 11, 2023
