Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**Posology and method of administration** Vectibix treatment should be supervised by a physician experienced in the use of anti-cancer therapy. Evidence of wild-type _RAS_ ( _KRAS_ and _NRAS_) status is required before initiating treatment with Vectibix. Mutational status should be determined by an experienced laboratory using validated test methods for detection of _KRAS_ (exons 2, 3 and 4) and _NRAS_ (exons 2, 3 and 4) mutations. Posology The recommended dose of Vectibix is 6 mg/kg of bodyweight given once every two weeks. Modification of the dose of Vectibix may be necessary in cases of severe (≥ grade 3) dermatological reactions as follows: 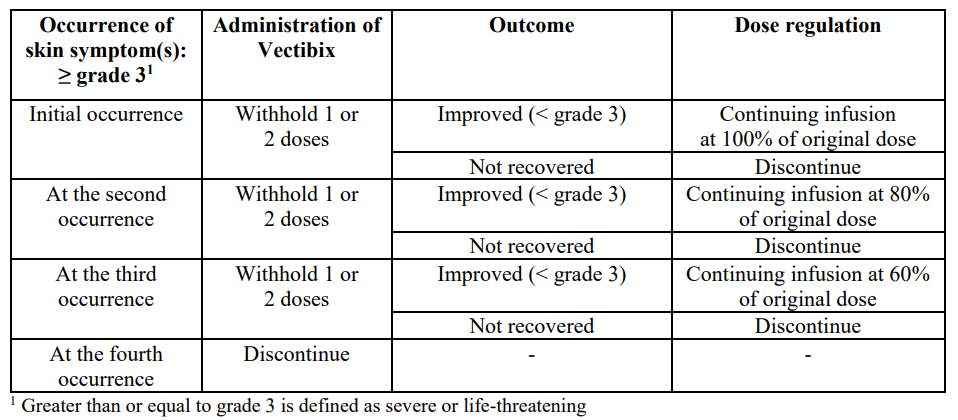 _Special populations_ The safety and efficacy of Vectibix have not been studied in patients with renal or hepatic impairment. There is no clinical data to support dose adjustments in the elderly. _Paediatric population_ There is no relevant use of Vectibix in the paediatric population in the indication treatment of colorectal cancer. Method of administration Vectibix must be administered as an intravenous infusion via an infusion pump. Prior to infusion, Vectibix should be diluted in sodium chloride 9 mg/mL (0.9%) solution for injection to a final concentration not to exceed 10 mg/mL (for preparation instructions see Special precautions for disposal and other handling section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Vectibix must be administered using a low protein binding 0.2 or 0.22 micrometre in-line filter, through a peripheral line or indwelling catheter. The recommended infusion time is approximately 60 minutes. If the first infusion is tolerated, then subsequent infusions may be administered over 30 to 60 minutes. Doses higher than 1,000 mg should be infused over approximately 90 minutes (for handling instructions, see Special precautions for disposal and other handling section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The infusion line should be flushed with sodium chloride solution before and after Vectibix administration to avoid mixing with other medicinal products or intravenous solutions. A reduction in the rate of infusion of Vectibix may be necessary in cases of infusion-related reactions (see Special warnings and precautions for use section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Vectibix must not be administered as an intravenous push or bolus. For instructions on dilution of the medicinal product before administration, see Special precautions for disposal and other handling section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
INTRAVENOUS
Medical Information
**Therapeutic indications** Vectibix is indicated for the treatment of adult patients with wild-type _RAS_ metastatic colorectal cancer (mCRC): - in first-line in combination with FOLFOX or FOLFIRI. - in second-line in combination with FOLFIRI for patients who have received first-line fluoropyrimidine-based chemotherapy (excluding irinotecan). - as monotherapy after failure of fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens.
**Contraindications** Patients with a history of severe or life-threatening hypersensitivity to the active substance or to any of the excipients listed in List of excipients section (see Special warnings and precautions for use section) – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. Patients with interstitial pneumonitis or pulmonary fibrosis (see Special warnings and precautions for use section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The combination of Vectibix with oxaliplatin-containing chemotherapy is contraindicated for patients with mutant _RAS_ mCRC or for whom _RAS_ mCRC status is unknown (see Special warnings and precautions for use section – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
L01XC08
xl 01 xc 08
Manufacturer Information
AMGEN BIOTECHNOLOGY SINGAPORE PTE. LTD.
Amgen Manufacturing Limited
Active Ingredients
Documents
Package Inserts
Vectibix Injection PI.pdf
Approved: May 4, 2022
