Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE, LIQUID FILLED
**4.2 DOSAGE AND ADMINISTRATION** **Adults** **Dosing in NSCLC** Treatment with OFEV should be initiated and supervised by a physician experienced in the use of anticancer therapies. For posology, method of administration, and dose modifications of docetaxel, please refer to the corresponding product information for docetaxel. The recommended dose of OFEV is 200 mg twice daily administered approximately 12 hours apart, on days 2 to 21 of a standard 21-day docetaxel treatment cycle. OFEV must not be taken on the same day of docetaxel chemotherapy administration (= day 1). The recommended maximum daily dose of 400 mg should not be exceeded. Patients may continue therapy with OFEV after discontinuation of docetaxel for as long as clinical benefit is observed or until unacceptable toxicity occurs. **Dosing in IPF / chronic fibrosing ILDs with a progressive phenotype / SSc-ILD** Treatment with OFEV should be initiated by physicians experienced in the diagnosis and treatment of conditions for which OFEV is indicated. The recommended dose of OFEV is 150 mg twice daily administered approximately 12 hours apart. The recommended maximum daily dose of 300 mg should not be exceeded. **Dose adjustments** _NSCLC_ As initial measure for the management of adverse reactions (see Tables 1a and 1b), treatment with nintedanib should be temporarily interrupted until the specific adverse reaction has resolved to levels that allow continuation of therapy (to grade 1 or baseline). Nintedanib treatment may be resumed at a reduced dose. Dose adjustments in 100 mg steps per day (i.e. a 50 mg reduction per dosing) based on individual safety and tolerability are recommended as described in Table 1a and Table 1b. In case of further persistence of the adverse reaction(s), i.e. if a patient does not tolerate 100 mg twice daily, treatment with OFEV should be permanently discontinued. In case of specific elevations of aspartate aminotransferase (AST)/ alanine aminotransferase (ALT) values to > 3 x upper limit normal (ULN) in conjunction with an increase of total bilirubin to ≥ 2 x ULN and alkaline phosphatase (ALKP) < 2 x ULN; (see Table 1b) treatment with OFEV should be interrupted. Unless there is an alternative cause established, OFEV should be permanently discontinued (see section _**Special Warnings and Precautions**_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). 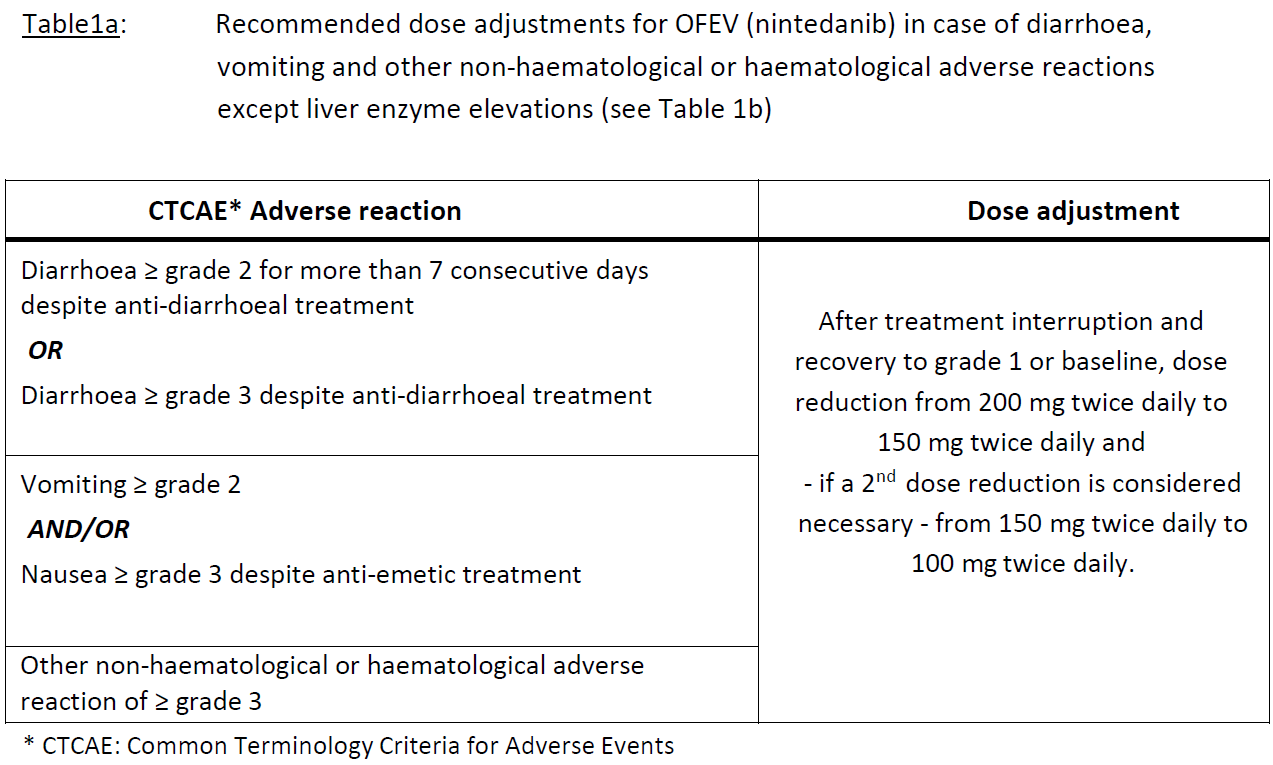 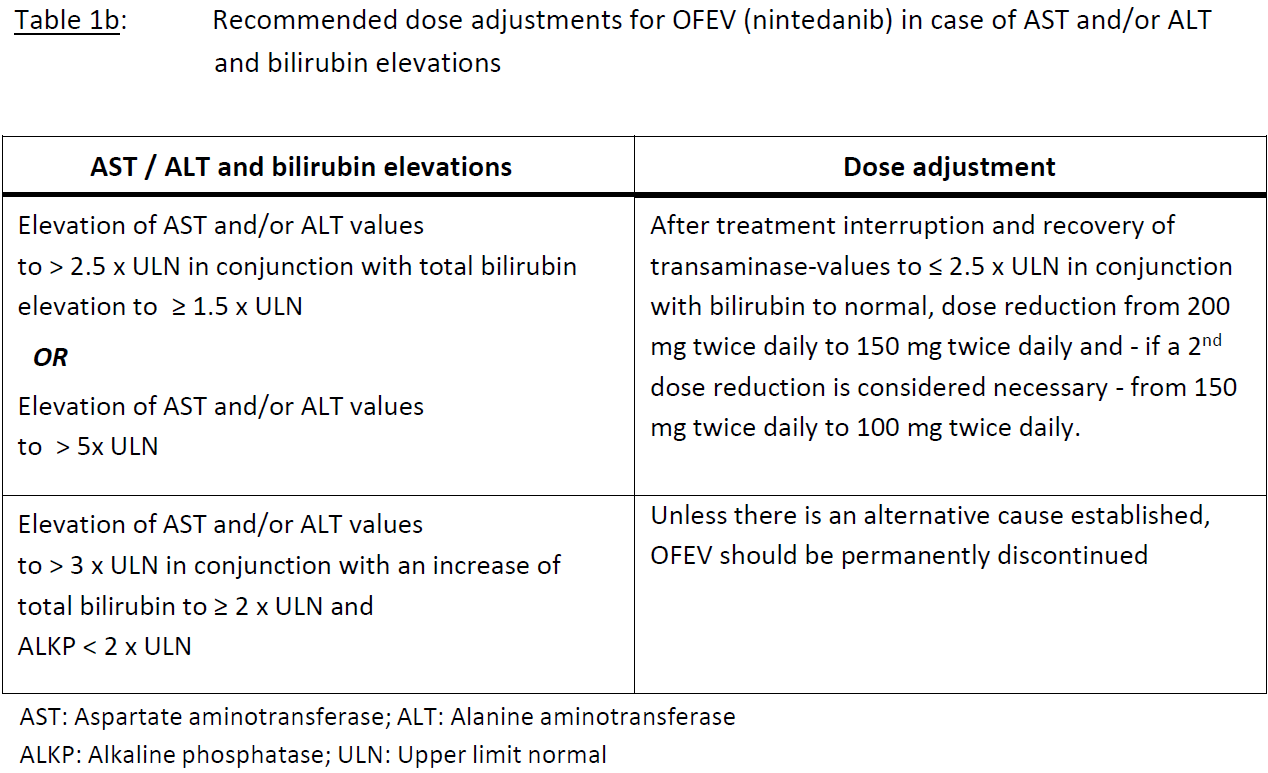 _IPF / chronic fibrosing ILDs with a progressive phenotype / SSc-ILD_ In addition to symptomatic treatment if applicable, the management of adverse reactions (see section **_Special Warnings and Precautions, Adverse Reactions_** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) of OFEV may require dose reduction or temporary interruption until the specific adverse reaction resolves to levels that allow continuation of therapy. OFEV treatment may be resumed at the full dosage (150 mg twice daily) or at the reduced dosage (100 mg twice daily), which subsequently may be increased to the full dosage. If a patient does not tolerate 100 mg twice daily, treatment with OFEV should be discontinued. Dose modifications or interruptions may be necessary for liver enzyme elevations. For aspartate aminotransferase (AST) or alanine aminotransferase (ALT) > 3 times to < 5 times the upper limit of normal (ULN) without signs of severe liver damage, interrupt treatment or reduce OFEV to 100mg twice daily. Once liver enzymes have returned to baseline values, treatment with OFEV may be reintroduced at a reduced dosage (100mg twice daily), which subsequently may be increased to the full dosage (150mg twice daily) \[ **_see section Special Warnings and Precautions, Adverse Reactions_** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\]. **Special populations** Paediatric population The safety and efficacy of OFEV in children aged 0–18 years have not been established. Elderly patients (≥ 65 years) No overall differences in safety and efficacy were observed for elderly patients. No adjustment of the initial dosing is required on the basis of a patient’s age (see section **_Pharmacokinetics_** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients ≥ 75 years may be more likely to require dose reduction to manage adverse effects. Race Based on population pharmacokinetic (-PK) analyses, no a priori dose adjustments of OFEV are necessary (see section Special Populations, **_Special Warnings and Precautions, Pharmacokinetics_** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Safety data for Black patients are limited. Body weight Based on population PK analyses, no a priori dose adjustments of OFEV are necessary (see section **_Pharmacokinetics_** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Renal impairment Less than 1% of a single dose of nintedanib is excreted via the kidney (see section **_Pharmacokinetics_** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Adjustment of the starting dose in patients with mild to moderate renal impairment is not required. The safety, efficacy, and pharmacokinetics of nintedanib have not been studied in patients with severe renal impairment (< 30 ml/min CrCL). Hepatic Impairment _IPF / chronic fibrosing ILDs with a progressive phenotype / SSc-ILD_ Nintedanib is predominantly eliminated via biliary/faecal excretion (> 90 %). Exposure increased in patients with hepatic impairment (Child Pugh A, Child Pugh B; see section **_Pharmacokinetics_** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In patients with mild hepatic impairment (Child Pugh A), the recommended dose of OFEV is 100mg twice daily approximately 12 hours apart. In patients with mild hepatic impairment (Child Pugh A), treatment interruption or discontinuation for management of adverse reactions should be considered. The safety and efficacy of nintedanib have not been investigated in patients with hepatic impairment classified as Child Pugh B and C. Treatment of patients with moderate (Child Pugh B) and severe (Child Pugh C) hepatic impairment with OFEV is not recommended (see section **_Pharmacokinetics_** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _NSCLC_ Nintedanib is predominantly eliminated via biliary/faecal excretion (> 90 %). Exposure increased in patients with hepatic impairment (Child Pugh A, Child Pugh B; see section _**Pharmacokinetics**_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). No adjustment of the starting dose is needed for patients with mild hepatic impairment (Child Pugh A) based on clinical data. Limited safety data available from 9 patients with moderate hepatic impairment (Child Pugh B) are insufficient to characterize this population. The safety, efficacy and pharmacokinetics of nintedanib have not been investigated in patients with severe hepatic impairment (Child Pugh C). Treatment of patients with moderate (Child Pugh B) and severe (Child Pugh C) hepatic impairment with OFEV is not recommended (see sections _**Special Warnings and Precautions for Use**_ and _**Pharmacokinetics**_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Method of Administration** OFEV capsules should be taken orally, preferably with food, swallowed whole with water, and should not be chewed. If a dose is missed, administration should resume at the next scheduled time at the recommended dose. If a dose is missed, the patient should not be given an additional dose. OFEV capsules may be taken with a small amount (teaspoonful) of cold or room temperature soft food, such as apple sauce or chocolate pudding, and must be swallowed unchewed immediately, to ensure the capsule stays intact. The capsule should not be opened or crushed. If contact with the content of the capsule occurs, hands should be washed immediately and thoroughly.
ORAL
Medical Information
**4.1 INDICATIONS** OFEV is indicated in combination with docetaxel for the treatment of adult patients with locally advanced, metastatic or locally recurrent non-small cell lung cancer (NSCLC) of adenocarcinoma tumour histology after first line chemotherapy. OFEV is indicated for the treatment of Idiopathic Pulmonary Fibrosis (IPF). OFEV is indicated for the treatment of chronic fibrosing Interstitial Lung Diseases (ILDs) with a progressive phenotype \[see section Clinical Trials – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\] OFEV is indicated to slow the rate of decline in pulmonary function in patients with systemic sclerosis associated interstitial lung disease (SSc-ILD).
**4.3 CONTRAINDICATIONS** OFEV is contraindicated in patients with known hypersensitivity to nintedanib, peanut or soya, or to any of the excipients (see section _**Composition**_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). OFEV is contraindicated during pregnancy (see sections _**Fertility, Pregnancy and Lactation and Toxicology**_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _NSCLC_ For contraindications of docetaxel please refer to the corresponding product information for docetaxel.
L01XE31
xl 01 xe 31
Manufacturer Information
BOEHRINGER INGELHEIM SINGAPORE PTE. LTD.
Catalent Germany Eberbach GmbH
Boehringer Ingelheim Pharma GmbH & Co. KG (Primary and Secondary Packager)
