Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, FOR SOLUTION
**4.2 Posology and method of administration** Posology Adults 1. Blood pool scintigraphy: The average activity administered after intravenous injection for _in vivo_ or after _in vitro_ labelling is 890 MBq (740–925 MBq). 2. Determination of blood volume: The average activity administered after intravenous injection after _in vitro_ labelling is 3 MBq (1–5 MBq). 3. Spleen scintigraphy: The average activity administered after intravenous injection after _in vitro_ labelling of denaturated erythrocytes is 50 MBq (20–70 MBq). The optimal amount of nonradioactive stannous tin for preparation of red blood cells (RBCs) _in vivo_ or _in vitro_ is 10 to 20 mcg/kg body weight in adults. Especially in cases of _in vitro_ labelling this dose should not be exceeded. Sodium pertechnetate (99mTc) should be injected ( _in vivo_) or added to the incubation mixture ( _in vitro_) after 30 minutes. Renal impairment Careful consideration of the activity to be administered is required since an increased radiation exposure is possible in these patients. Paediatric population The use in children and adolescents has to be considered carefully, based upon clinical needs and assessing the risk/benefit ratio in this patient group. The activities to be administered to children and to adolescents may be calculated according to the EANM dosage card version 2016 for the indication: Blood pool scintigraphy. _A\[MBq\]Administered = 56.0 x Multiple from table 1_ Spleen scintigraphy: _A\[MBq\]Administered = 2.8 x Multiple from table 1_ 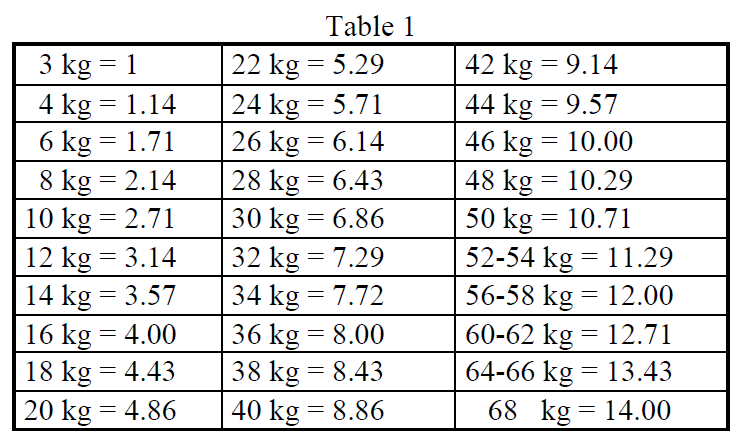 For blood scintigraphy, in very young infants (up to 1 year) a minimum dose of 80 MBq is necessary in order to obtain images of sufficient quality. For spleen scintigraphy a minimum dose of 20 MBq is necessary. Method of administration Multidose vial. For intravenous injection. This medicinal product should be reconstituted before administration to the patient. For instructions regarding reconstitution, see section 12 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. For patient preparation, see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The freeze-dried stannous pyrophosphate lyophilizate (non-radioactive substance) is first reconstituted with isotonic sodium chloride solution for injection. _In vivo RBCs labelling method:_ Injection of the reconstituted solution of the stannous pyrophosphate complex followed by injection of sodium pertechnetate (99mTc) 30 minutes later. _In vitro RBCs labelling method:_ - Sampling of 6 ml of the patient’s blood - _In vitro_ incubation of the reconstituted solution of the taken total blood sample or separated RBCs, followed by adding sodium pertechnetate (99mTc) 30 minutes later. - _Second in vitro_ incubation of the RBCs and reinjection of the labelled RBCs 30 minutes later. _Modified in vivo RBCs labelling method (in vivo/in vitro):_ - Injection of the reconstituted solution of the stannous pyrophosphate for _in vivo_ “stannous-loading” of RBCs. - _In vitro_ RBCs labeling with sodium pertechnetate (99mTc) after taking a blood sample. - Reinjection of the labelled RBCs. _Denatured RBCs labelling method:_ - _In vitro_ labelling of RBCs (see above) followed by denaturation e.g. heating of the labelled erythrocytes at 49–50°C for 25 minutes. - Reinjection of the labelled denatured RBCs. Image acquisition _Angiocardioscintigraphy:_ The acquisition of images starts immediately after the injection of the tracer. _Occult digestive haemorrhages:_ Since digestive bleeding occurs usually intermittently, it is recommended to perform several acquisitions over a period of 24 hours in addition to the images acquired initially after the injection. _Spleen scintigraphy:_ Images are performed from 30 to 120 minutes after the injection. In case of accessory spleen research, the entire abdomen should be studied. If the patient has diaphragm rupture due to previous trauma, the chest should also be studied.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** This medicinal product is for diagnostic use only. 1. Red blood cell labelling for blood pool scintigraphy. Major indications are: - Angiocardioscintigraphy for: \*evaluation of ventricular ejection fraction \*evaluation of global and regional cardiac wall motion \*myocardial phase imaging - Organ perfusion and vascular abnormalities imaging for the detection of hemangioma. - Diagnosis and localization of occult gastro-intestinal bleeding 2. Determination of blood volume 3. Spleen scintigraphy
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
V09BA03
technetium (99mTc) pyrophosphate
Manufacturer Information
QT INSTRUMENTS (S) PTE LTD
Curium Netherlands B.V.
