Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
DOSAGE AND ADMINISTRATION: **Adult patients with newly diagnosed glioblastoma multiforme** TEMODAL is administered in combination with focal radiotherapy (concomitant phase) followed by up to 6 cycles of temozolomide monotherapy. _**Concomitant phase**_ TEMODAL is administered orally at 75 mg/m2 daily for 42 days concomitant with focal radiotherapy (60 Gy administered in 30 fractions). No dose reductions are recommended, but delay or discontinuation of temozolomide administration will be decided weekly according to haematological and non-haematological toxicity criteria. The TEMODAL dose can be continued throughout the 42 day concomitant period up to 49 days if all of the following conditions are met: absolute neutrophil count ≥ 1.5 x 109/L; thrombocyte count ≥ 100 x 109/L; Common Toxicity Criteria (CTC) non-hematological toxicity ≤ Grade 1 (except for alopecia, nausea and vomiting). During treatment a complete blood count should be obtained weekly. TEMODAL dosing should be interrupted or discontinued during concomitant phase according to the hematological and non-hematological toxicity criteria as noted in **Table 1**. 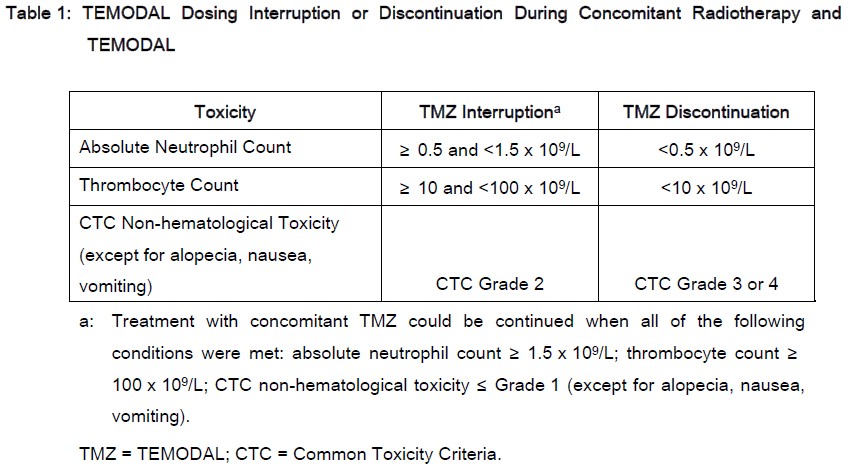 _**Monotherapy Phase**_ Four weeks after completing the TEMODAL + Radiotherapy phase, TEMODAL is administered for up to 6 cycles of monotherapy treatment. Dosage in Cycle 1 (monotherapy) is 150 mg/m2 once daily for 5 days followed by 23 days without treatment. At the start of Cycle 2, the dose is escalated to 200 mg/m2 if the CTC non-hematologic toxicity for Cycle 1 is Grade ≤ 2 (except for alopecia, nausea and vomiting), absolute neutrophil count (ANC) is ≥ 1.5 x 109/L, and the thrombocyte count is ≥ 100 x 109/L. If the dose was not escalated at Cycle 2, escalation should not be done in subsequent cycles. Once escalated, the dose remains at 200 mg/m2 per day for the first 5 days of each subsequent cycle except if toxicity occurs. Dose reductions and discontinuations during the monotherapy phase should be applied according to **Tables 2 and 3**. During treatment a complete blood count should be obtained on day 22 (21 days after the first dose of TEMODAL). The TEMODAL dose should be reduced or discontinued according to **Table 3**. 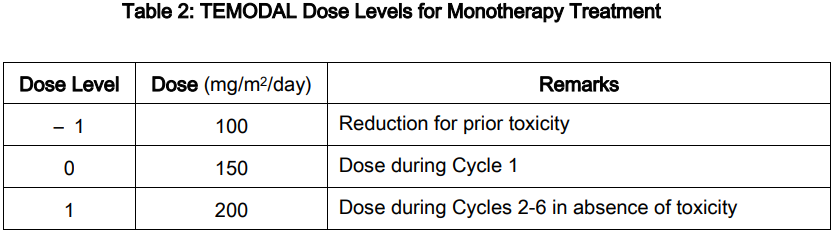 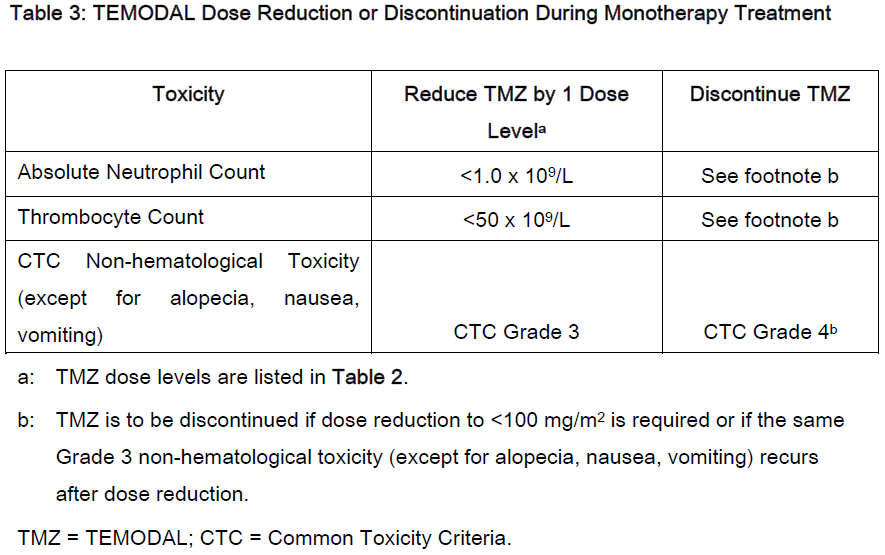 Adult with recurrent or progressive glioma: In patients previously untreated with chemotherapy, TEMODAL is administered orally at a dose of 200 mg/m2 once daily for 5 days per 28-day cycle. In patients previously treated with chemotherapy, the initial dose is 150 mg/m2 once daily, to be increased in the second cycle to 200 mg/m2 daily providing the absolute neutrophil count (ANC) is ≥ 1.5 x 109/L and the thrombocyte count is ≥ 100 x 109/L on Day 1 of the next cycle. Dose modification for TEMODAL should be based on toxicities according to nadir ANC or platelet counts. Pediatric patients with recurrent or progressive glioma: In patients 3 years of age or older, TEMODAL is administered orally at a dose of 200 mg/m2 once daily for 5 days per 28-day cycle. Pediatric patients previously treated with chemotherapy should receive an initial dose of 150 mg/m2 once daily for 5 days, with escalation to 200 mg/m2 once daily for 5 days at the next cycle if there is no toxicity. Therapy can be continued until disease progression for a maximum of 2 years. Laboratory parameters for dose modification in recurrent or progressive malignant glioma: Prior to dosing, the following laboratory parameters must be met: absolute neutrophil count (ANC) ≥ 1.5 x 109/L and platelets ≥ 100 x 109/L. A complete blood count must be obtained on Day 22 (21 days after the first dose) or within 48 hours of that day, and weekly until ANC is above 1.5 x 109/L and platelet count exceeds 100 x 109/L. If the ANC falls to < 1.0 x 109/L or the platelet count is < 50 x 109/L during any cycle, the next cycle should be reduced one dose level. Dose levels include 100 mg/m2, 150 mg/m2, and 200 mg/m2. The lowest recommended dose is 100 mg/m2. All Patients: TEMODAL should be administered in the fasting state, at least one hour before a meal. Antiemetic therapy may be administered prior to or following administration of TEMODAL. If vomiting occurs after the dose is administered, a second dose should not be administered that day. TEMODAL Capsules must not be opened or chewed, but are to be swallowed whole with a glass of water. If a capsule becomes damaged, avoid contact of the powder contents with skin or mucous membrane.
ORAL
Medical Information
INDICATIONS AND USAGE: TEMODAL Capsules are indicated for the treatment of patients with: - newly diagnosed glioblastoma multiforme concomitantly with radiotherapy and subsequently as monotherapy treatment. - malignant glioma, such as glioblastoma multiforme or anaplastic astrocytoma, showing recurrence or progression after standard therapy.
CONTRAINDICATIONS: TEMODAL is contraindicated in patients who have a history of hypersensitivity reaction to its components or to dacarbazine (DTIC) since both drugs are metabolized to MTIC. TEMODAL is contraindicated for use during pregnancy or breast feeding (see USAGE DURING PREGNANCY AND LACTATION – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). TEMODAL is contraindicated in patients with severe myelosuppression.
L01AX03
temozolomide
Manufacturer Information
MSD PHARMA (SINGAPORE) PTE. LTD.
Organon Heist bv (Primary and secondary packaging)
ORION CORPORATION ORION PHARMA
Active Ingredients
Documents
Package Inserts
Temodal PI_Approved.pdf
Approved: July 28, 2022
