Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2.2 DOSAGE AND ADMINISTRATION** _**General**_ Cotellic therapy should only be initiated and supervised by a healthcare professional experienced in the treatment of patients with cancer. Patients treated with Cotellic in combination with vemurafenib must have BRAF V600 mutation-positive melanoma tumor status confirmed by a validated test. Please also refer to the full prescribing information for vemurafenib, which is used in combination with Cotellic. _Standard Dosage_ The recommended dose of Cotellic is 60 mg (three 20 mg tablets) once daily. Cotellic is taken on a 28 day cycle. Each Cotellic dose consists of three 20 mg tablets (60mg) and should be taken once daily for 21 consecutive days (days 1 to 21 – treatment period); followed by a 7 day break in Cotellic treatment (days 22 to 28 – treatment break). Each dose of three 20 mg tablets (60 mg) can be taken with or without food ( _see Section 3.2.1 Absorption_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Cotellic tablets should be swallowed whole with water. _Duration of treatment_ Treatment with Cotellic should continue until the patient no longer derives benefit or until the development of unacceptable toxicity. _Delayed or Missed doses_ If a dose is missed, it can be taken up to 12 hours prior to the next dose to maintain the once-daily regimen. _Vomiting_ In case of vomiting after Cotellic administration, the patient should not take an additional dose of Cotellic on that day, and treatment should be continued as prescribed the following day. _**Dose modification recommendations**_ _**General**_ Cotellic dose modification should be based on the prescriber's assessment of individual patient safety or tolerability. If doses are omitted for toxicity; missed doses should not be replaced. Once the dose has been reduced, it should not be increased at a later time. Dose modification of Cotellic is independent of vemurafenib dose modification. The decision on whether to dose reduce either or both drugs should be based on clinical assessment. Table 1 below gives general Cotellic dose modification advice. 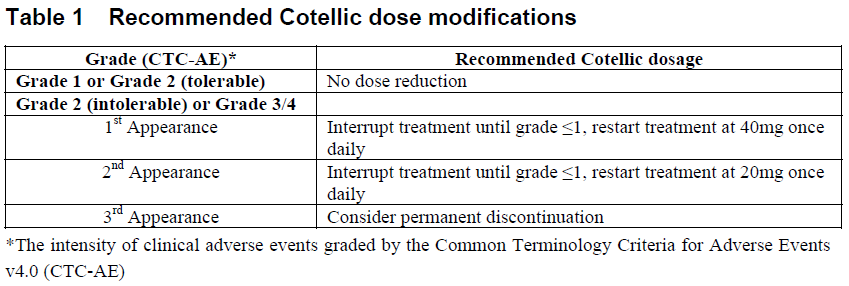 _**Dose modification advice for specified adverse drug reactions(ADRs)**_ _**Hemorrhage**_ Grade 4 events or cerebral hemorrhage (all grades): Interrupt Cotellic treatment. Permanently discontinue Cotellic for hemorrhage events attributed to Cotellic. Grade 3 events: Interrupt Cotellic treatment. There is no data on the effectiveness of Cotellic dose modification for hemorrhage events. Clinical judgment should be applied when considering restarting Cotellic treatment. Vemurafenib dosing can be continued when Cotellic treatment is interrupted, if clinically indicated. _**Left ventricular dysfunction**_ Permanent discontinuation of Cotellic treatment should be considered if cardiac symptoms are attributed to Cotellic and do not improve after temporary interruption of Cotellic. 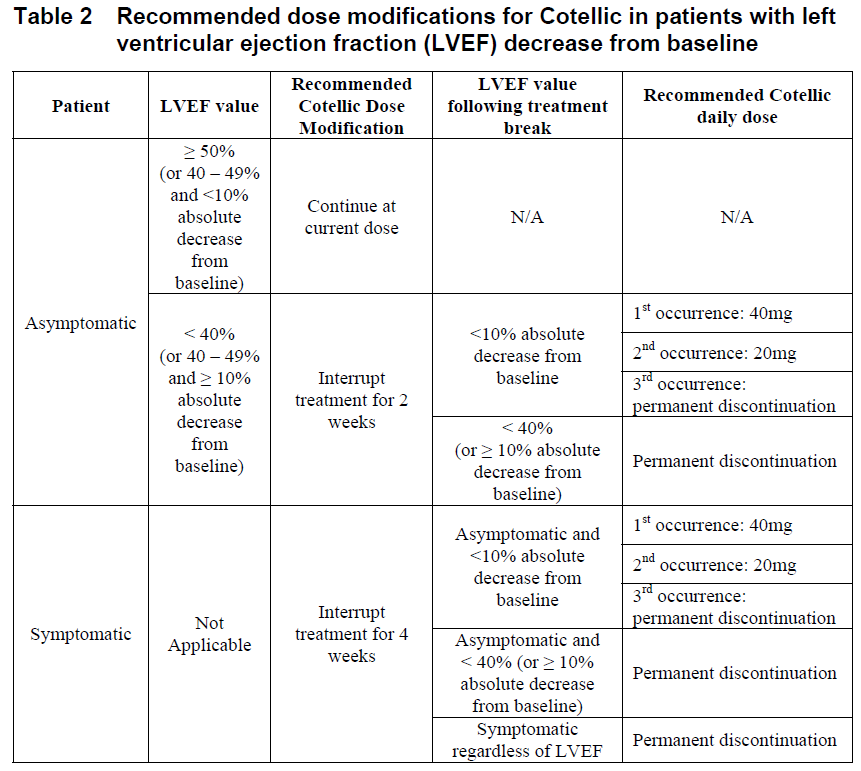 Vemurafenib treatment can be continued when Cotellic treatment is modified (if clinically indicated). _**Rhabdomyolysis and Creatine phosphokinase (CPK) elevations**_ _Rhabdomyolysis or symptomatic CPK elevations:_ Interrupt Cotellic treatment. If severity is improved by at least one grade within 4 weeks, restart Cotellic at a dose reduced by 20 mg, if clinically indicated. Vemurafenib dosing can be continued when Cotellic treatment is modified, if clinically indicated. If rhabdomyolysis or symptomatic CPK elevations do not improve within 4 weeks, permanently discontinue Cotellic treatment. _Asymptomatic CPK elevations:_ Grade ≤ 3: Cotellic dosing does not need to be modified or interrupted to manage asymptomatic Grade ≤ 3 creatine phosphokinase (CPK) elevations ( _see section 2.6 Undesirable Effects, Clinical Trials, Laboratory Abnormalities_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Grade 4: Interrupt Cotellic treatment. If improved to Grade ≤ 3 within 4 weeks, restart Cotellic at a dose reduced by 20 mg, if clinically indicated. Vemurafenib dosing can be continued when Cotellic treatment is modified, if clinically indicated. If CPK elevations do not improve to Grade ≤3 within 4 weeks following dose interruption, permanently discontinue Cotellic treatment. _**Liver laboratory abnormalities**_ For Grade ≤ 2 liver laboratory abnormalities, Cotellic and vemurafenib should be continued at the prescribed dose. Grade 3: Continue Cotellic at the prescribed dose. The dose of vemurafenib may be reduced as clinically appropriate. Please refer to the full prescribing information for vemurafenib. Grade 4: Interrupt Cotellic treatment and vemurafenib treatment. If liver laboratory abnormalities improve to Grade ≤1 within 4 weeks, restart Cotellic at a dose reduced by 20 mg and vemurafenib at a clinically appropriate dose; please refer to the full prescribing information for vemurafenib. If liver laboratory abnormalities do not resolve to Grade ≤1 within 4 weeks or if Grade 4 liver laboratory abnormalities recur, discontinue Cotellic treatment and vemurafenib treatment. _**Photosensitivity**_ Grade ≤ 2 (tolerable) photosensitivity should be managed with supportive care. Grade 2 (intolerable) or Grade ≥ 3 photosensitivity: Cotellic and vemurafenib should be interrupted until resolution to Grade ≤ 1. Treatment can be restarted with no change in Cotellic dose. Vemurafenib dosing should be reduced; please refer to the full prescribing information for vemurafenib. _**Rash**_ Rash events may occur with either Cotellic or vemurafenib treatment. The dose of Cotellic and/or vemurafenib may be either interrupted and/or reduced as clinically indicated. Additionally: Grade ≤ 2 (tolerable) rash should be managed with supportive care. Grade 2 (intolerable) or Grade ≥ 3 rash: Acneiform rash: Follow general dose modification table recommendations in Table 1 for Cotellic. Vemurafenib dosing can be continued when Cotellic treatment is modified (if clinically indicated). Non-acneiform or maculopapular rash: Cotellic dosing can be continued without modification (if clinically indicated). Vemurafenib dosing may be either temporarily interrupted and/or reduced, please refer to the full prescribing information for vemurafenib. **QT prolongation** If during treatment the QTc exceeds 500 msec, please refer to the vemurafenib product insert for dose modifications for vemurafenib. No dose modification of Cotellic is required when taken in combination with vemurafenib. **2.2.1 Special Dosage Instructions** _Elderly:_ No dose adjustment of Cotellic is required in patients ≥ 65 years of age. _Children:_ The safety and efficacy of Cotellic in children and adolescents (<18 years) have not been established. (see sections 2.5.4 Pediatric Use, 3.1.2 Clinical/Efficacy studies and 3.2.5 Pharmacokinetics in Special Populations – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment:_ No dose adjustment is recommended in patients with mild or moderate renal impairment, based on population pharmacokinetic analysis. The safety and efficacy of Cotellic have not been established in patients with severe renal impairment ( _see section 3.2.5 Pharmacokinetics in Special Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Hepatic impairment:_ No dose adjustment is recommended in patients with hepatic impairment ( _see section 3.2.5 Pharmacokinetics in Special Populations_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Cotellic should be used with caution in patients with moderate to severe hepatic impairment. Liver laboratory abnormalities can occur when Cotellic is used in combination with vemurafenib ( _see section 2.4 Warnings and Precautions, General_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
ORAL
Medical Information
**2.1 THERAPEUTIC INDICATION(S)** Cotellic is indicated for use in combination with vemurafenib for the treatment of patients with unresectable or metastatic melanoma with BRAF V600 mutation.
**2.3 CONTRAINDICATIONS** Cotellic is contraindicated in patients with known hypersensitivity to cobimetinib or any of the excipients.
LO1XE
xlo 1 xe
Manufacturer Information
ROCHE SINGAPORE PTE. LTD.
F. Hoffmann-La Roche Ltd
Delpharm Milano S.r.l. (Primary & Secondary Packager)
Excella GmbH & Co. KG
Active Ingredients
Documents
Package Inserts
Cotellic film-coated tablet 20mg PI.pdf
Approved: November 8, 2017
