Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**Recommended Dosage:** The rapid injection of a large volume of local anesthetic solution should be avoided and fractional (incremental) doses should always be used. The smallest dose and concentration required to produce the desired result should be administered. The dose of any local anesthetic differs with the anesthetic procedure, the area to be anesthetized, the vascularity of the tissues, the number of neuronal segments to be blocked, the intensity of the block, the degree of muscle relaxation required, the duration of the anesthesia desired, individual tolerance, and the physical condition of the patient. Patients in poor general condition due to aging or other compromising factors, such as impaired cardiovascular function, advanced liver disease, or severe renal dysfunction, require special attention. To reduce the risk of potentially serious adverse reactions, attempts should be made to optimize the patient's condition before major blocks are performed, and the dosage should be adjusted accordingly. Use an adequate test dose (3 to 5 ml) of a short-acting local anesthetic solution containing epinephrine prior to induction of complete nerve block. This test dose should be repeated if the patient is moved in such a fashion as to have displaced the epidural catheter. It is recommended that adequate time be allowed for the onset of anesthesia following administration of each test dose. The use of levobupivacaine is not recommended for more than 24 hours (see **Warnings and Precautions** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Disinfecting agents containing heavy metals, which cause release of ions (mercury, zinc, copper, etc.) should not be used for skin or mucous membrane disinfection since they have been related to incidents of swelling and edema. When chemical disinfection of the container surface is desired, either isopropyl alcohol (91%) or ethyl alcohol (70%) is recommended. It is recommended that chemical disinfection be accomplished by wiping the ampule thoroughly with cotton or gauze that has been moistened with the recommended alcohol prior to use. These products are intended for single use and do not contain preservatives; any solution remaining from an open container should be discarded. 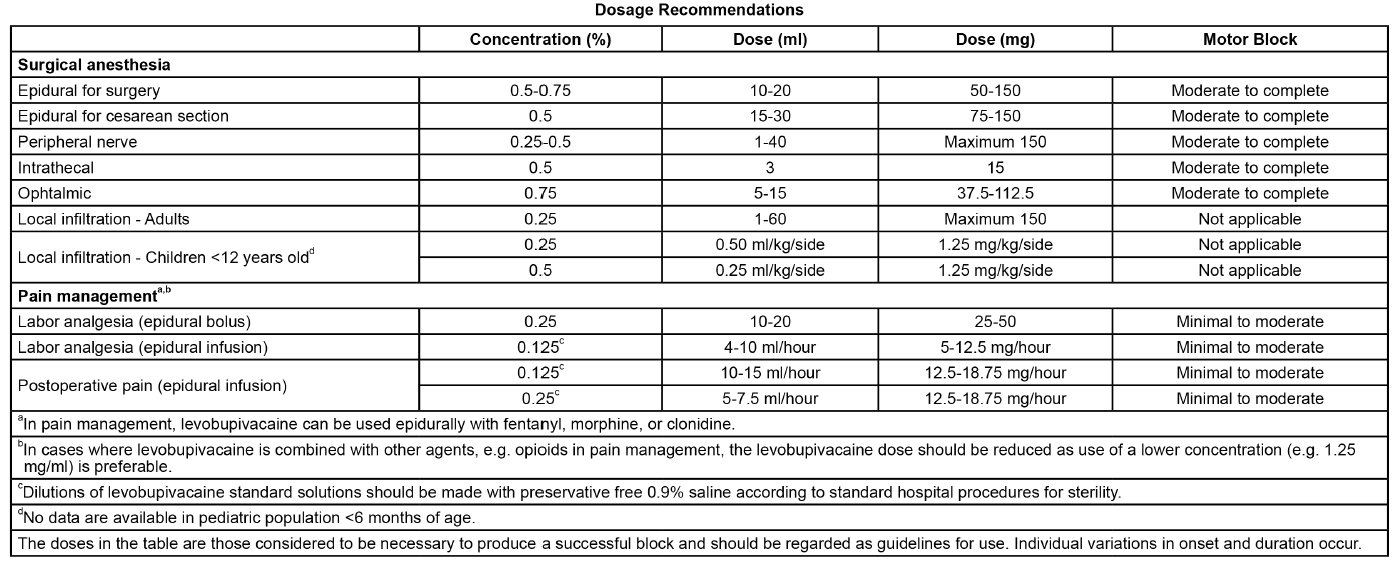 Epidural doses of up to 375 mg have been administered incrementally to patients during a surgical procedure. The maximum dose in 24 hours for intraoperative block and postoperative pain management was 695 mg. The maximum dose administered as a postoperative epidural infusion over 24 hours was 570 mg. The maximum dose administered to patients as a single fractionated injection was 300 mg for brachial plexus block. For cesarean section, the maximum recommended dose is 150 mg. In children, the maximum recommended dose for infiltration analgesia (ilioinguinal-iliohypogastric block) is 1.25 mg/kg/side.
EPIDURAL, INTRATHECAL
Medical Information
**Indications:** _**Adults**_ Levobupivacaine is indicated in adults for: - Surgical anesthesia Major: epidural (including for cesarean section), intrathecal, peripheral nerve block. Minor: local infiltration, peribulbar block in ophthalmic surgery. For cesarean section, the 7.5 mg/ml concentration is not recommended (see **Contraindications, Warnings and Precautions** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Pain management Continuous epidural infusion, single or multiple bolus administration for postoperative, labor or chronic pain. For continuous epidural analgesia, levobupivacaine may be administered in combination with epidural fentanyl, morphine or clonidine. For labor analgesia, the 7.5 mg/ml concentration is not recommended (see **Contraindications, Warnings and Precautions, Use during Pregnancy and Lactation– _Labor and Delivery_** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Children**_ Levobupivacaine is indicated in children for infiltration analgesia (ilioinguinal/iliohypogastric blocks) (see **Recommended Dosage**).
**Contraindications:** - General contraindications related to regional anesthesia should be taken into account with the use of any regional anesthetic agent, including levobupivacaine. Levobupivacaine solutions are contraindicated in those with a known sensitivity to local anesthetic amide agents. - Levobupivacaine is contraindicated in patients with severe hypotension such as cardiogenic or hypovolemic shock (see **Warnings and Precautions** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Levobupivacaine solutions are contraindicated for use in paracervical block in obstetrics, and for intravenous regional anesthesia (e.g. Bier’s block). - Additionally, levobupivacaine 7.5 mg/ml solution should not be employed for any obstetric procedures. Contraindications for use in paracervical block, Bier’s block, and levobupivacaine 7.5 mg/ml use in obstetric procedures are based upon documented experiences with bupivacaine. Levobupivacaine has not been tested in such instances.
N01BB10
levobupivacaine
Manufacturer Information
GLORIOUS DEXA SINGAPORE PTE. LTD.
PT. Ferron Par Pharmaceuticals
Active Ingredients
Documents
Package Inserts
L-Ascain_PI.pdf
Approved: February 9, 2023
