Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
POWDER, FOR SOLUTION
**4.2. Posology and method of administration** Premedication with a corticosteroid, antihistamine, and acetaminophen (or paracetamol) is recommended 1 hour prior to MYLOTARG dosing to help ameliorate infusion-related symptoms (see Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Appropriate measures to help prevent the development of tumor lysis-related hyperuricemia such as hydration, administration of antihyperuricemic or other agents for treatment of hyperuricemia must be taken (see Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients with hyperleukocytosis (leukocyte count >30,000/mm3), cytoreduction is recommended prior to administration of MYLOTARG (see Table 2). MYLOTARG must be reconstituted and diluted before administration (see Section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Posology _Newly-diagnosed de novo CD33-positive AML (combination regimen)_ _Patients 15 years and above_ The recommended dose of MYLOTARG is 3 mg/m2. A treatment course including MYLOTARG in combination therapy with newly-diagnosed de novo CD33-positive AML consists of 1 induction cycle and 2 consolidation cycles. _Induction_ The recommended dose of MYLOTARG is 3 mg/m2/dose (up to a maximum of one 5 mg vial) infused over a 2-hour period on Days 1, 4, and 7 in combination with daunorubicin (DNR) 60 mg/m2/day infused over 30 minutes on Day 1 to Day 3, and cytarabine (AraC) 200 mg/m2/day by continuous infusion on Day 1 to Day 7. If a second induction is required, MYLOTARG should not be administered during second induction therapy. Only DNR and AraC should be administered during the second induction cycle, at the following recommended dosing: DNR 35 mg/m2/day on Days 1 and 2, and AraC 1 g/m2 every 12 hours, on Day 1 to Day 3. _Consolidation_ For patients experiencing a complete remission (CR) following induction, defined as fewer than 5% blasts in a normocellular marrow and an absolute neutrophil count (ANC) of more than 1.0 × 109 cells/L with a platelet count of 100 × 109/L or more in the peripheral blood in the absence of transfusion, up to 2 consolidation courses of intravenous DNR (60 mg/m2 for 1 day \[first course\] or 2 days \[second course\]) in combination with intravenous AraC (1 g/m2 per 12 hours, infused over 2 hours on Day 1 to Day 4) with intravenous MYLOTARG (3 mg/m2/dose infused over 2 hours up to a maximum dose of one 5 mg vial on Day 1) are recommended. Table 1 shows dosing regimens for MYLOTARG in combination with chemotherapy. 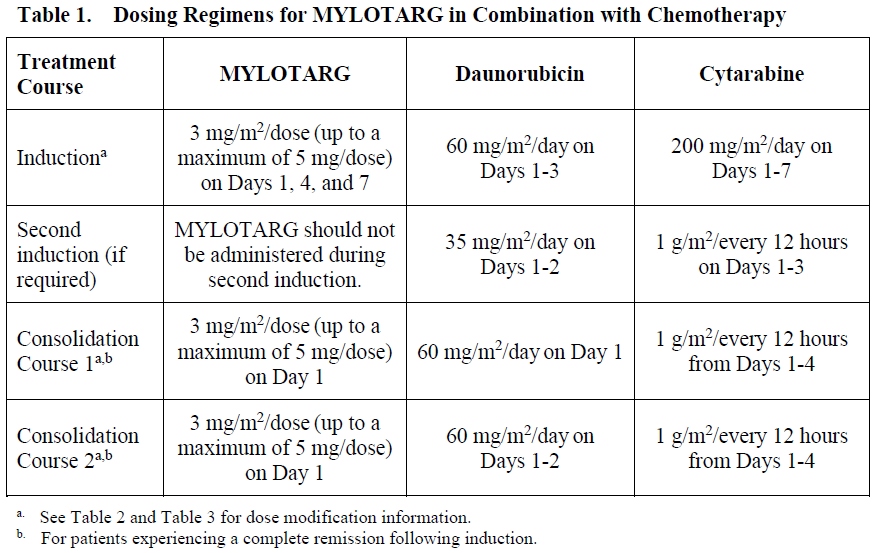 _Pediatric patients (1 month and older)_ The recommended dose of MYLOTARG in pediatric patients 1 month and older with previously untreated de novo AML is: - 3 mg/m2 for patients with body surface area (BSA) greater than or equal to 0.6 m2 - 0.1 mg/kg for patients with BSA less than 0.6 m2 For Induction 1, MYLOTARG is given once in combination with standard chemotherapy. No MYLOTARG is given in the second induction cycle. No MYLOTARG is given in the first or third intensification cycles. For Intensification 2, MYLOTARG is given once in combination with standard chemotherapy. Consider the risks and potential benefits before giving MYLOTARG during Intensification 2. Dose and schedule modifications _Schedule modification for hyperleukocytosis_ In patients with hyperleukocytic (leukocyte count >30,000/mm3) AML, cytoreduction is recommended either with leukapheresis, oral hydroxyurea (previously untreated AML), or AraC with or without hydroxyurea (previously untreated AML) to reduce the peripheral white blood cell (WBC) count 48 hours prior to administration of MYLOTARG (see Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If AraC is used for leukoreduction with or without hydroxyurea in patients with previously untreated, de novo hyperleukocytic AML receiving MYLOTARG in combination therapy, apply the following modified schedule (Table 2): 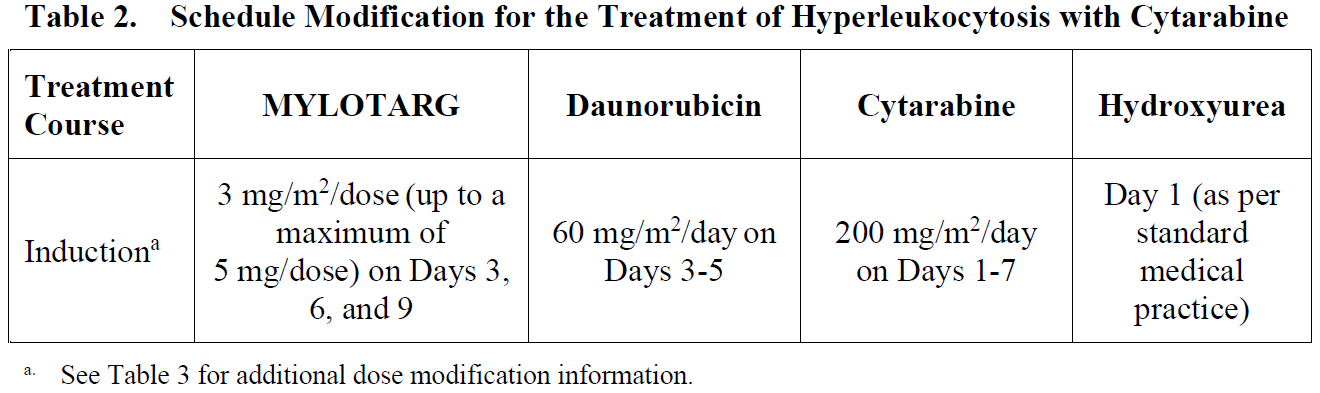 _Dose modification for adverse drug reactions_ Dose modification of MYLOTARG is recommended based on individual safety and tolerability (see Section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Management of some adverse drug reactions may require dose interruptions or permanent discontinuation of MYLOTARG (see Sections 4.4 and 4.8 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Table 3 shows the dose modification guidelines for hematologic and nonhematologic toxicities. 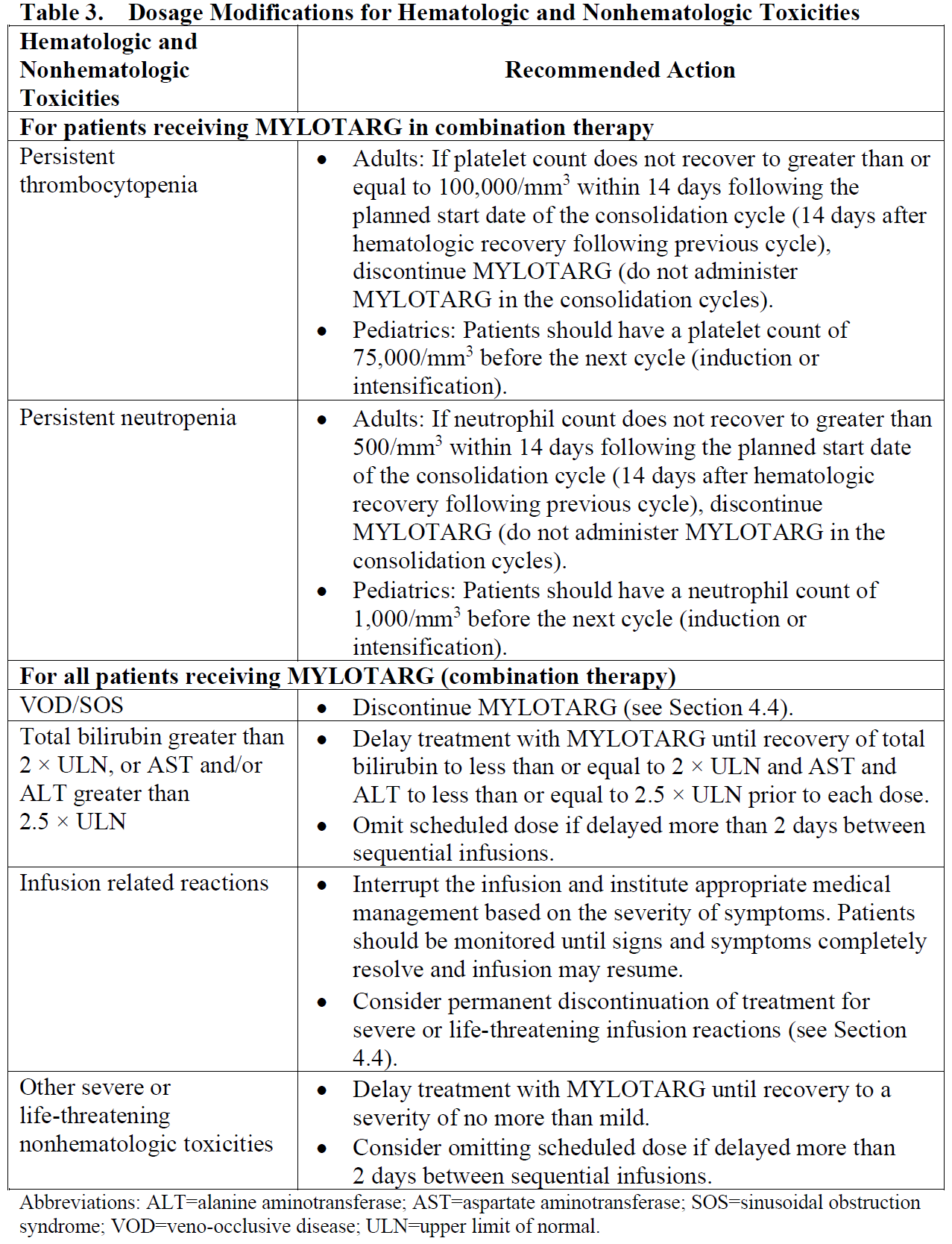 Special populations _Use in patients with hepatic impairment_ No adjustment to dose of MYLOTARG is required in patients with hepatic impairment defined by total bilirubin ≤ 2 × upper limit of normal (ULN) and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 2.5 × ULN. Postpone MYLOTARG until recovery of total bilirubin to ≤ 2 × ULN and AST and ALT to ≤ 2.5 × ULN prior to each dose (see Table 3 and Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). MYLOTARG has not been studied in patients with severe hepatic impairment. _Use in patients with renal impairment_ No adjustment to dose of MYLOTARG is required in patients with mild to moderate renal impairment. MYLOTARG has not been studied in patients with severe renal impairment. _Elderly patients_ No adjustment to dose of MYLOTARG is required in elderly patients (≥65 years) (see Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Pediatric population_ The safety and efficacy of MYLOTARG in combination with chemotherapy in the pediatric population (<1 month) with newly-diagnosed AML have not been established. Method of administration Administer MYLOTARG intravenously by infusion over a 2-hour period under close clinical monitoring, including pulse, blood pressure, and temperature. Do not administer MYLOTARG as an intravenous push or bolus (see Section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
INTRAVENOUS
Medical Information
**4.1. Therapeutic indications** MYLOTARG is indicated for the treatment of newly-diagnosed, de novo CD33-positive acute myeloid leukemia in adults and pediatric patients 1 month and older, except acute promyelocytic leukemia (APL) (see Sections 4.4 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3. Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in Section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L01XC05
xl 01 xc 05
Manufacturer Information
PFIZER PRIVATE LIMITED
Wyeth Pharmaceutical Division of Wyeth Holdings LLC
Active Ingredients
Documents
Package Inserts
Mylotarg PI.pdf
Approved: June 27, 2022
