Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SUSPENSION
**3.2 Posology and method of administration** The vaccination schedule depends on the age of the subject. 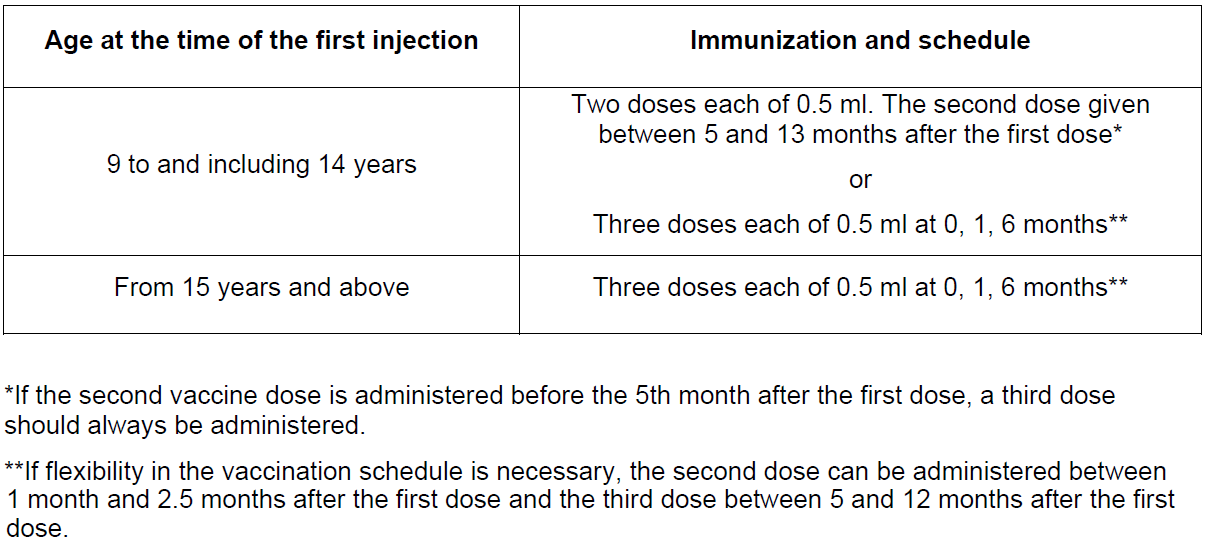 The necessity for a booster dose has not been established _(see section 4.1_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_. Cervarix is for intramuscular injection in the deltoid region. Cervarix should under no circumstances be administered intravascularly or intradermally. No data are available on subcutaneous administration of Cervarix. Paediatric population: Cervarix is not recommended for use in children below 9 years of age.
INTRAMUSCULAR
Medical Information
**3.1 Therapeutic indications** Cervarix is a vaccine indicated in females from 9 to 25 years of age for the prevention of persistent infection, premalignant cervical lesions and cervical cancer caused by Human Papillomavirus (HPV) Types 16 & 18. See sections 3.4 and 4.1 for important information on the data regarding HPV-16 and/or HPV-18, and other oncogenic HPV types that support this indication – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The indication is based on the demonstration of efficacy in women aged 15 to 25 years following vaccination with Cervarix and on the immunogenicity of the vaccine in girls and women aged 9 to 25 years. The use of Cervarix should be in accordance with official recommendations.
**3.3 Contraindications** Cervarix should not be administered to subjects with known hypersensitivity to any component of the vaccine _(see sections 2 and 5.1_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _)_. Individuals who develop symptoms indicative of hypersensitivity after receiving a dose of Cervarix should not receive further doses of Cervarix.
J07BM02
papillomavirus (human types 16, 18)
Manufacturer Information
GLAXOSMITHKLINE PTE LTD
GlaxoSmithKline Biologicals s.a.
Active Ingredients
Documents
Package Inserts
Cervarix PI.pdf
Approved: May 26, 2023
