Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**2 DOSAGE AND ADMINISTRATION** **2.1 Recommended Dosage** The recommended dosage of BRUKINSA is 160 mg taken orally twice daily or 320 mg taken orally once daily until disease progression or unacceptable toxicity. BRUKINSA can be taken with or without food. Advise patients to swallow capsules whole with water. Advise patients not to open, break, or chew the capsules. If a dose of BRUKINSA is missed, it should be taken as soon as possible on the same day with a return to the normal schedule the following day. **2.2 Dosage Modification for Use in Hepatic Impairment** The recommended dosage of BRUKINSA for patients with severe hepatic impairment is 80 mg orally twice daily _\[see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **2.3 Dosage Modifications for Drug Interactions** Recommended dosage modifications of BRUKINSA for drug interactions are provided in Table 1 _\[see Drug Interactions (7.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.  After discontinuation of a CYP3A inhibitor or moderate CYP3A4 inducer, resume previous dose of BRUKINSA _\[see Dosage and Administration (2.1, 2.2) and Drug Interactions (7.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **2.4 Dosage Modifications for Adverse Reactions** Recommended dosage modifications of BRUKINSA for Grade 3 or higher adverse reactions are provided in Table 2: 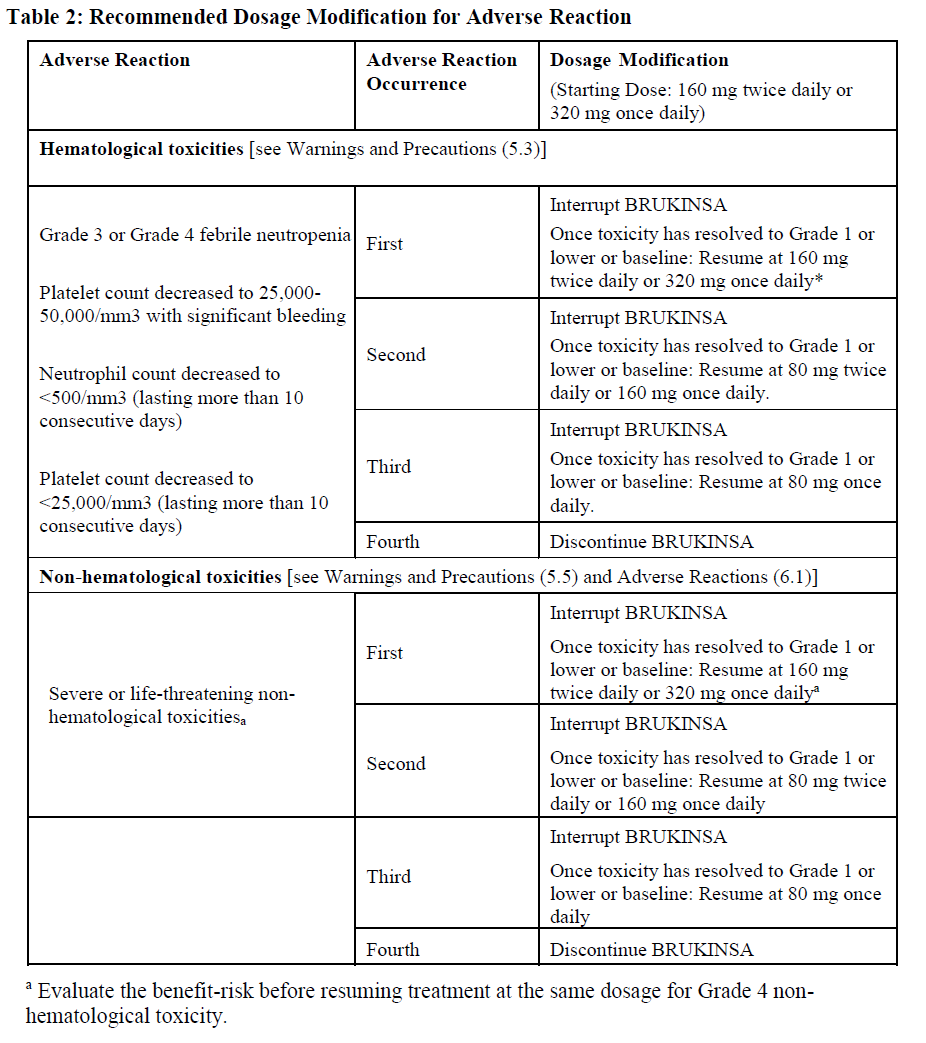 Asymptomatic lymphocytosis should not be regarded as an adverse reaction, and these patients should continue taking BRUKINSA.
ORAL
Medical Information
**1 INDICATIONS AND USAGE** **1.1 Mantle Cell Lymphoma** BRUKINSA is indicated for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy. **1.2 Waldenström’s Macroglobulinemia** BRUKINSA as monotherapy is indicated for the treatment of adult patients with Waldenström’s macroglobulinemia (WM) who have received at least one prior therapy, or in first line treatment for patients unsuitable for chemo-immunotherapy _\[see Clinical Studies (14.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **1.3 Marginal Zone Lymphoma** BRUKINSA as monotherapy is indicated for the treatment of adult patients with relapsed or refractory marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based regimen _\[see Clinical Studies (14.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **1.4 Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma** BRUKINSA is indicated for the treatment of adult patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) _\[see Clinical Studies (14.4)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
**4 CONTRAINDICATIONS** None.
L01EL03
zanubrutinib
Manufacturer Information
BEIGENE SINGAPORE PTE. LTD.
Catalent CTS (Kansas City), LLC (Primary packager)
Active Ingredients
Documents
Package Inserts
Brukinsa capsule Package Insert.pdf
Approved: October 10, 2022
