Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**2\. DOSAGE AND ADMINISTRATION** **2.1. Initiation, Maintenance, and Interruption of Treatment** Confirm absence of pregnancy and usage of effective contraception in females of reproductive potential _\[see Warnings and Precautions (5.4)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Initiation or up-titration of CAMZYOS in patients with LVEF <55% is not recommended. The recommended starting dose is 5 mg once daily without regard to food; allowable subsequent doses with titration are 2.5, 5, 10, or 15 mg once daily. Patients may develop heart failure while taking CAMZYOS. Regular LVEF and Valsalva left ventricular outflow tract (LVOT) gradient assessment is required for careful titration to achieve an appropriate target Valsalva LVOT gradient, while maintaining LVEF ≥50% and avoiding heart failure symptoms (see Figure 1 and Figure 2). Additional assessment of LVEF is recommended if clinical status changes or in patients with a serious intercurrent illness such as infection or arrhythmia (including atrial fibrillation or other uncontrolled tachyarrhythmia). Daily dosing takes weeks to reach steady-state drug levels and therapeutic effects, and genetic variation in metabolism and drug interactions can cause large differences in exposure _\[see Contraindications (4), Warnings and Precautions (5.2), Drug Interactions (7.1) and Clinical Pharmacology (12.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. When initiating or titrating CAMZYOS, first consider LVEF then consider the Valsalva LVOT gradient and patient clinical status to guide appropriate CAMZYOS dosing. Follow the algorithms for Initiation (Figure 1) and Maintenance (Figure 2) for appropriate CAMZYOS dosing and monitoring schedules. If LVEF <50% while taking CAMZYOS, interrupt treatment. Follow the algorithm for Interruption (Figure 3) for guidance on interrupting, restarting, or discontinuing CAMZYOS. If interrupted at 2.5 mg, either restart at 2.5 mg or discontinue permanently. 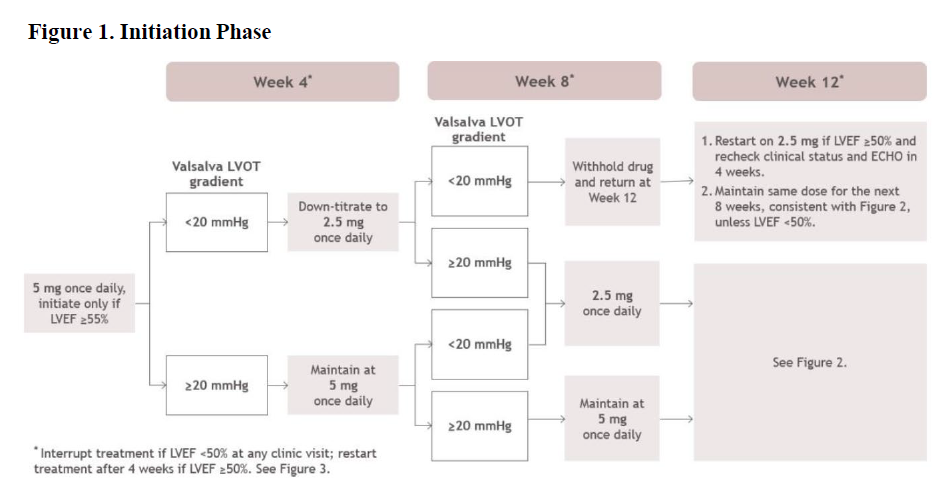 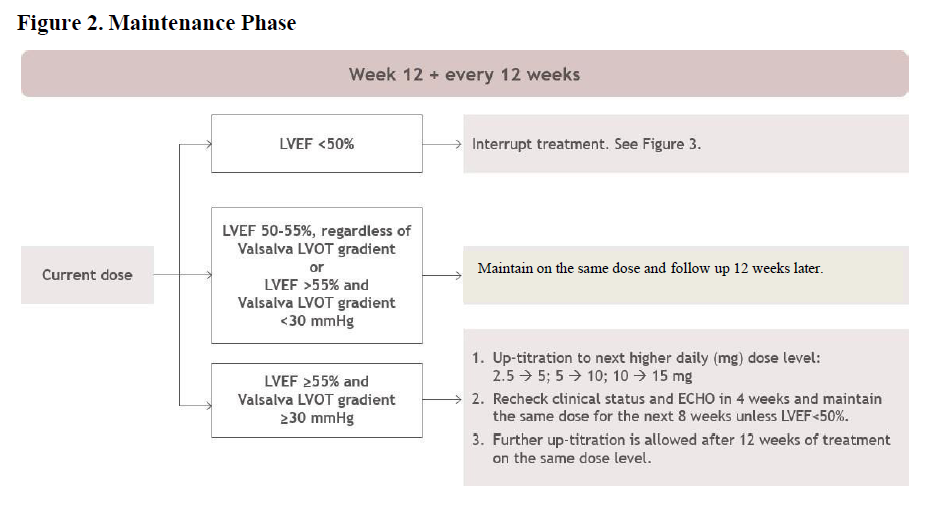 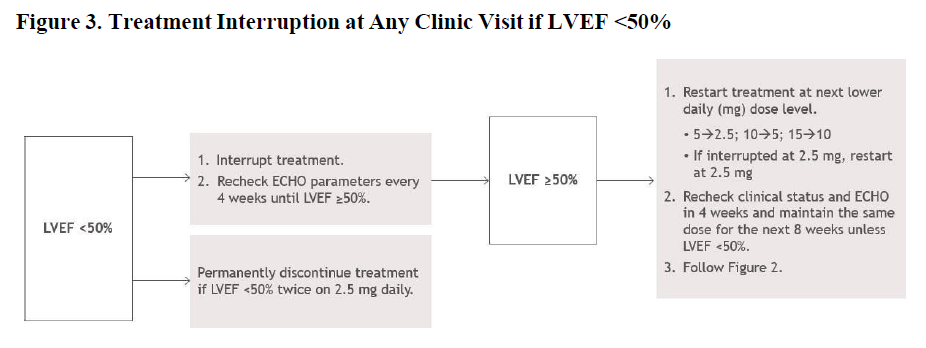 Delay dose increases when there is intercurrent illness (e.g., serious infection) or arrhythmia (e.g., atrial fibrillation or other uncontrolled tachyarrhythmia) that may impair systolic function. Consider interruption of CAMZYOS in patients with intercurrent illness _\[see Warnings and Precautions (5.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Missed or delayed doses If a dose is missed, it should be taken as soon as possible, and the next scheduled dose should be taken at the usual time the following day. Exact timing of dosing during the day is not essential, but two doses should not be taken on the same day. Swallow capsules whole. Do not break, open, or chew the capsules. **2.2. Concomitant Administration of Weak CYP2C19 or Moderate CYP3A4 Inhibitors** Initiate CAMZYOS at the recommended starting dosage of 5 mg orally once daily in patients who are on stable therapy with a weak CYP2C19 inhibitor or a moderate CYP3A4 inhibitor. Reduce dosage of CAMZYOS by one level (i.e., 15 🡺 10 mg; 10 🡺 5 mg; or 5 🡺 2.5 mg) in patients who initiate a weak CYP2C19 inhibitor or a moderate CYP3A4 inhibitor. Schedule clinical and echocardiographic assessment 4 weeks after inhibitor initiation, and do not up-titrate CAMZYOS until 12 weeks after inhibitor initiation. Avoid initiation of concomitant weak CYP2C19 and moderate CYP3A4 inhibitors in patients who are on stable treatment with 2.5 mg of CAMZYOS because a lower CAMZYOS once-daily dose is not available _\[see Dosage and Administration (2.1), Drug Interactions (7.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
ORAL
Medical Information
**1\. INDICATIONS AND USAGE** CAMZYOSTM is indicated for the treatment of adults with symptomatic New York Heart Association (NYHA) class II–III obstructive hypertrophic cardiomyopathy (HCM) to improve functional capacity and symptoms.
**4\. CONTRAINDICATIONS** CAMZYOS is contraindicated with concomitant use of: - Moderate to strong CYP2C19 inhibitors or strong CYP3A4 inhibitors _\[see Warnings and Precautions (5.2), Drug Interactions (7.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_ - Moderate to strong CYP2C19 inducers or moderate to strong CYP3A4 inducers _\[see Warnings and Precautions (5.2), Drug Interactions (7.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_
C01EB24
mavacamten
Manufacturer Information
LIANBIO DEVELOPMENT (SG) PTE. LTD.
Patheon Inc.
AndersonBrecon Inc. (Primary packager and secondary packager)
Active Ingredients
Documents
Package Inserts
Camzyos Capsule PI.pdf
Approved: June 14, 2023
