Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** WARNING: The different low molecular weight heparins are not necessarily equivalent. Therefore, compliance with the dosage regimen and the specific method of use for each of these medicinal products is required. Posology _Adults_ _Total knee arthroplasty with high risk of venous thromboembolism:_ On the day of the surgical procedure, 3,500 international units anti-Xa is to be administered by sc route, 6 hours after surgery. On subsequent days, 3,500 international units anti-Xa sc is to be administered every 24 hours. Prophylactic treatment must be followed in accordance with the physician’s opinion, during the period of risk or until the patient is completely mobilised. As a general rule, it is considered necessary to maintain prophylactic treatment for at least 7 – 10 days after the surgical procedure and until the risk of thromboembolic disease has decreased. _Paedriatic population_ HIBOR is not recommended for use in children under 18 years due to a lack of data on safety and efficacy. _Elderly_ No dose adjustment is required, unless renal function is altered (see section 4.2 _Posology and method of administration,_ Renal impairment; 4.4 _Special warnings and precautions for use;_ 5.2 _Pharmacokinetic properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Renal impairment_ (See sections 4.4 _Special warnings and precautions for use_ and 5.2 _Pharmacokinetic properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - For daily doses of bemiparin 3500 international units: - In patients with mild or moderate renal insufficiency (creatinine clearance 30–80 ml/min): no dose adjustment is necessary. However, a close monitoring is recommended. - In patients with severe renal insufficiency (creatinine clearance <30 ml/min) could influence the pharmacokinetics of bemiparin. Physicians should assess the individual bleeding and thrombotic risks in these patients. In some cases, the dose may be necessary to be adjusted. Based on limited pharmacokinetic data (See Section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_), a dose reduction up to 2,500 international units anti-Xa s.c. once daily could be recommended. A close monitoring is recommended. Measurement of peak anti-Xa levels at about 4 hours post-dose should be considered. _Hepatic impairment_ The use of bemiparin has not been studied in patients with hepatic impairment and therefore is not recommended. Method of administration _Subcutaneous injection technique:_ You should follow these steps: - Wash your hands thoroughly. The patient should be sitting or lying in a comfortable position at the time of Hibor administration. - The administration of HIBOR by subcutaneous route is performed by injecting the syringe in the subcutaneous cell tissue of the anterolateral or posterolateral abdominal waist, to 5 centimetres from the navel and any scar or bruise. Clean the skin in that area. - Use different places for the injection on different days, for example, first on the left hand side, next time on the right. - Pull the needle cap off the HIBOR syringe. 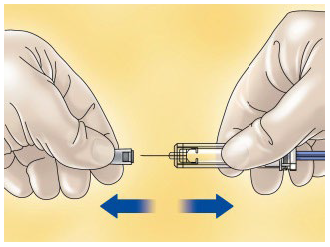 - To keep the needle sterile, make sure it doesn´t touch anything. - The pre-filled syringe is now ready for use. - Before injecting, do not push the plunger to get rid of any air bubbles, because you might lose the medicine. 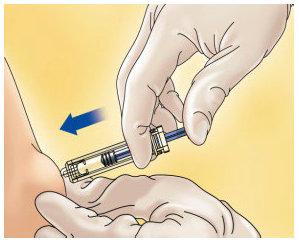 - Hold the syringe in one hand and with your other hand, using your forefinger and thumb, gently pinch the area of skin which you’ve cleaned and make a skin fold. - Insert the whole needle into the folded skin keeping the syringe as straight as possible on the body surface at a 90º angle. - Press down on the plunger, making sure you hold the skin fold in position throughout the injection. 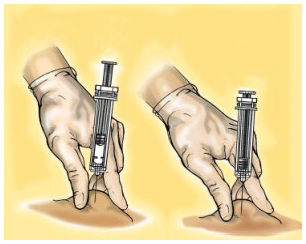 - Remove the syringe from the injection site keeping your finger on the plunger rod and the syringe straight. Let go of the skin fold. 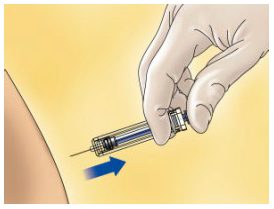 - Immediately dispose the syringe throwing it into the closest sharp object´s bin (the needle pointing inside), close the container lid tightly and place it out of reach of children. 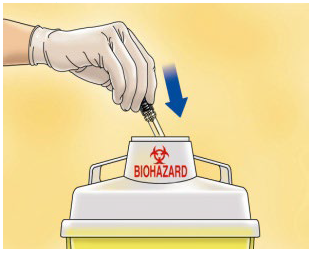 Warnings: - Do not reuse the needle shield after injection. - Do not rub the skin at the injection site. This will help to avoid bruises.
SUBCUTANEOUS
Medical Information
**4.1 Therapeutic indications** Prevention of thromboembolic disease in patients (at high risk of developing VTE) undergoing total knee arthroplasty.
**4.3 Contraindications** Hypersensitivity to sodium bemiparin, heparin or substances of porcine origin or to any of the excipients included on section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. History of confirmed or suspected immunologically mediated heparin induced thrombocytopenia (HIT) (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Active haemorrhage or increased risk of bleeding due to alterations of haemostasis. Severe impairment of liver or pancreatic function. Injuries or surgical interventions on the central nervous system, eyes and ears within the last 2 months. Disseminated Intravascular Coagulation (DIC) attributable to heparin-induced thrombocytopenia. Acute bacterial endocarditis and slow endocarditis. Any organic lesion susceptible of bleeding (e.g.: active peptic ulcer, haemorrhagic stroke, cerebral aneurysm or cerebral neoplasms).
B01AB12
bemiparin
Manufacturer Information
DCH AURIGA SINGAPORE
ROVI PHARMA INDUSTRIAL SERVICES, S.A.
Active Ingredients
Documents
Package Inserts
Hibor SmPC 2500&3500_SGBEM10000280322_clean.pdf
Approved: April 1, 2022
