Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**Dosage and Administration** Ondansetron is available for oral and parenteral use to allow the route of administration and dosing to be flexible. However this product is for intravenous or intramuscular use only. _Chemotherapy and radiotherapy induced nausea and vomiting (CINV and RINV)_ The emetogenic potential of cancer treatment varies according to the doses and combinations of chemotherapy and radiotherapy regimens used. The selection of dose regimen should be determined by the severity of the emetogenic challenge. - **CINV and RINV in Adults** The recommended intravenous (IV) or intramuscular (IM) dose of ondansetron is 8 mg administered immediately before treatment. - Highly emetogenic chemotherapy A single dose of ondansetron 8 mg by slow intravenous injection immediately before chemotherapy has been shown to be effective in many patients. For highly emetogenic chemotherapy, a maximum initial ondansetron dose of 16 mg IV infused over 15 minutes may be used. A single IV dose greater than 16 mg should not be given due to dose- dependent increase of QT prolongation risk (see Warnings and Precautions, Adverse Reactions, Pharmacodynamic Effects – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). The efficacy of ondansetron in highly emetogenic chemotherapy may be enhanced by the addition of a single IV dose of dexamethasone sodium phosphate, 20 mg administered prior to chemotherapy. IV doses greater than 8 mg and up to a maximum of 16 mg must be diluted in 50 mL to 100 mL of 0.9% Sodium Chloride Injection or 5% Dextrose Injection before administration and infused over not less than 15 minutes (see _Special precautions for disposal and other handling_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Ondansetron doses of 8 mg ondansetron or less, do not need to be diluted and may be administered as a slow IM or IV injection in not less than 30 seconds. The initial dose of Ondansetron B. Braun may be followed by 2 additional IV or IM doses of 8 mg no less than four hours apart. Oral treatment is recommended to protect against delayed or prolonged emesis after the first 24 hours. - **CINV in children and adolescents (aged 2 years and over)** The dose for CINV can be calculated based on body surface area (BSA) or weight – see below. Ondansetron injection should be diluted in 5 % glucose or 0.9 % sodium chloride or other compatible infusion fluid and infused intravenously over not less than 15 minutes. There are no data from controlled clinical trials on the use of ondansetron in the prevention of delayed or prolonged CINV in children. There are no data from controlled clinical trials on the use of ondansetron for radiotherapy-induced nausea and vomiting in children. Dosing by BSA: Ondansetron should be administered immediately before chemotherapy as a single intravenous dose of 5 mg/m2. The intravenous dose must not exceed 8 mg. Oral dosing can commence twelve hours later and may be continued for up to 5 days (Table 1). 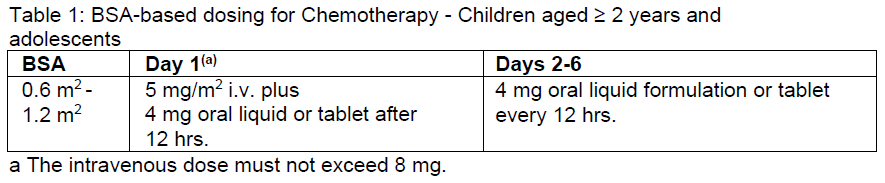 - **CINV and RINV in Elderly** In patients 65 to 74 years of age, the initial IV dose of Ondansetron 8 mg or 16 mg, infused over 15 minutes, may be followed by 2 doses of 8 mg infused over 15 minutes and given no less than 4 hours apart. All IV doses should be diluted in 50 – 100 mL of saline or other compatible infusion fluid and infused over 15 minutes. In patients 75 years of age or older, the initial IV dose of ondansetron should not exceed 8 mg, infused over 15 minutes. The initial dose of 8 mg may be followed by 2 doses of 8 mg, infused over 15 minutes and given no less than 4 hours apart (see _Special Patient Population, Elderly_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). All IV doses should be diluted in 50 – 100 mL of saline or other compatible infusion fluid and infused over 15 minutes. - **Renal impairment** No alteration of daily dosage or frequency of dosing, or route of administration is required. - **Hepatic impairment** Clearance of ondansetron is significantly reduced and serum half life significantly prolonged in subjects with moderate or severe impairment of hepatic function. In such patients a total daily dose of 8 mg should not be exceeded. - **Patients with Poor Sparteine/Debrisoquine Metabolism** The elimination half-life of ondansetron is not altered in subjects classified as poor metabolisers of sparteine and debrisoquine. Consequently in such patients repeat dosing will give drug exposure levels not different from those of the general population. No alteration of daily dosage or frequency of dosing is required. POST-OPERATIVE NAUSEA AND VOMITING - **PONV in Adults** For the prevention of post-operative nausea and vomiting, the recommended dose of ondansetron is a single dose of 4 mg by IM or slow IV injection administered at the induction of anaesthesia. For treatment of established post-operative nausea and vomiting a single dose of 4 mg given by IM or slow IV injection is recommended. - **PONV in Children and Adolescents (aged 2 years and over)** For prevention of PONV in paediatric patients having surgery performed under general anaesthesia, ondansetron may be administered by slow IV injection (not less than 30 seconds) at a dose of 0.1 mg/kg up to a maximum of 4 mg either prior to, at or after induction of anaesthesia, or after surgery. There is limited data on the use of ondansetron in the prevention and treatment of PONV in children under 2 years of age. - **Elderly** There is limited experience in the use of ondansetron in the prevention and treatment of post-operative nausea and vomiting in the elderly. However ondansetron is well tolerated in patients over 65 years receiving chemotherapy. - **Renal Impairment** No alteration of daily dosage or frequency of dosing, or route of administration is required. - **Hepatic Impairment** Clearance of ondansetron is significantly reduced and serum half life significantly prolonged in subjects with moderate or severe impairment of hepatic function. In such patients a total daily dose of 8 mg should not be exceeded. - **Patients with Poor Sparteine/Debrisoquine Metabolism** The elimination half-life of ondansetron is not altered in subjects classified as poor metabolisers of sparteine and debrisoquine. Consequently in such patients repeat dosing will give drug exposure levels not different from those of the general population. No alteration of daily dosage or frequency of dosing is required.
INTRAVENOUS, INTRAMUSCULAR
Medical Information
**Indications** **Adults** Ondansetron B. Braun 2 mg/ml solution for injection or infusion is indicated for the management of nausea and vomiting induced by cytotoxic chemotherapy and radiotherapy. Ondansetron B. Braun 2 mg/ml solution for injection or infusion is also indicated for the prevention and treatment of post-operative nausea and vomiting **Paediatric population** Ondansetron B. Braun 2 mg/ml solution for injection or infusion is indicated for the management of nausea and vomiting induced by cytotoxic chemotherapy Ondansetron B. Braun 2 mg/ml solution for injection or infusion is also indicated for the prevention and treatment of postoperative nausea and vomiting
**Contraindications** Based on reports of profound hypotension and loss of consciousness when ondansetron was administered with apomorphine hydrochloride, concomitant use with apomorphine is contraindicated. Hypersensitivity to any components of the preparation.
A04AA01
ondansetron
Manufacturer Information
B. Braun Singapore Pte Ltd.
B. Braun Melsungen AG
Active Ingredients
Documents
Package Inserts
Ondansetron Injection PI.pdf
Approved: January 12, 2023
