Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION
**2 DOSAGE AND ADMINISTRATION** **2.1 Dosage and Administration Overview** - VEKLURY may only be administered in settings in which healthcare providers have immediate access to medications to treat a severe infusion or hypersensitivity reaction, such as anaphylaxis, and the ability to activate the emergency medical system (EMS), as necessary _\[see Dosage and Administration (2.6, 2.7), Warnings and Precautions (5.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. - Administer VEKLURY for the treatment of COVID-19 in adults and pediatric patients (28 days of age and older and weighing at least 3 kg) by intravenous infusion only. Do not administer by any other route. - VEKLURY for injection (supplied as 100 mg lyophilized powder in vial) must be reconstituted with Sterile Water for Injection prior to diluting with 0.9% sodium chloride injection. - Carefully follow the product-specific preparation instructions below _\[see Dosage and Administration (2.6, 2.7)\]_. **2.2 Testing Before Starting and During Treatment with VEKLURY** Determine eGFR in all patients before starting VEKLURY and monitor while receiving VEKLURY as clinically appropriate _\[see Dosage and Administration (2.4) and Use in Specific Populations (8.3, 8.5)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Perform hepatic laboratory testing in all patients before starting VEKLURY and while receiving VEKLURY as clinically appropriate _\[see Warnings and Precautions (5.2) and Use in Specific Populations (8.6)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Determine prothrombin time in all patients before starting VEKLURY and monitor while receiving VEKLURY as clinically appropriate _\[see Adverse Reactions (6.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **2.3 Recommended Dosage in Adults, Adolescent and Pediatric Patients 28 days of Age and Older and Weighing at Least 3 kg** The recommended dosage for adults and adolescent patients weighing at least 40 kg is a single loading dose of VEKLURY 200 mg on Day 1 via intravenous infusion followed by once-daily maintenance doses of VEKLURY 100 mg from Day 2 via intravenous infusion. The recommended dosage for pediatric patients 28 days of age and older and weighing 3 kg to less than 40 kg is a single loading dose of VEKLURY 5 mg/kg on Day 1 via intravenous infusion followed by once-daily maintenance doses of VEKLURY 2.5 mg/kg from Day 2 via intravenous infusion. 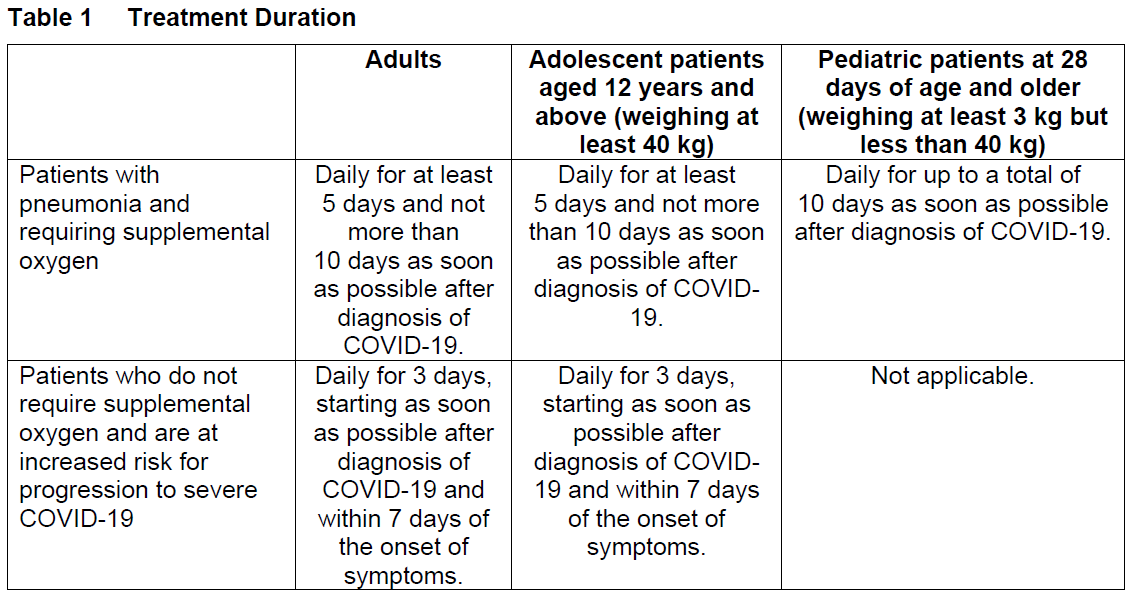 VEKLURY must be diluted prior to intravenous infusion. Refer to Dosage and Administration (2.6, 2.7) for detailed preparation and administration instructions. **2.4 Renal Impairment** VEKLURY is not recommended in patients with eGFR less than 30 mL per minute _\[see Dosage and Administration (2.2) and Use in Specific Populations (8.3, 8.5)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **2.5 Immunocompromised Population** The safety and efficacy of remdesivir in immunocompromised patients have not yet been established. Only limited data are available ( _see section 5.4_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **2.6 Dosage Preparation and Administration in Adults and Adolescent Patients Weighing at Least 40 kg** **Carefully follow the product-specific preparation instructions below.** Reconstitution Instructions Remove the required number of single-dose vial(s) from storage. For each vial: - Aseptically reconstitute VEKLURY lyophilized powder by adding 19 mL of Sterile Water for Injection using a suitably sized syringe and needle per vial. - Only use Sterile Water for Injection to reconstitute VEKLURY lyophilized powder. - Discard the vial if a vacuum does not pull the Sterile Water for Injection into the vial. - Immediately shake the vial for 30 seconds. - Allow the contents of the vial to settle for 2 to 3 minutes. A clear, colorless to yellow solution, free of visible particles, should result. - If the contents of the vial are not completely dissolved, shake the vial again for 30 seconds and allow the contents to settle for 2 to 3 minutes. Repeat this procedure as necessary until the contents of the vial are completely dissolved. Discard the vial if the contents are not completely dissolved. - Following reconstitution, each vial contains 100 mg/20 mL (5 mg/mL) of remdesivir solution. - Use reconstituted product immediately to prepare the diluted drug product _\[see Dosage and Administration (2.8)\]_. Dilution Instructions **Care should be taken during admixture to prevent inadvertent microbial contamination.** As there is no preservative or bacteriostatic agent present in this product, aseptic technique must be used in preparation of the final parenteral solution. It is always recommended to administer intravenous medication immediately after preparation when possible. - Reconstituted VEKLURY for injection, containing 100 mg/20 mL remdesivir solution, must be further diluted in either a 100 mL or 250 mL 0.9% sodium chloride injection infusion bag. Refer to Table 2 for instructions. 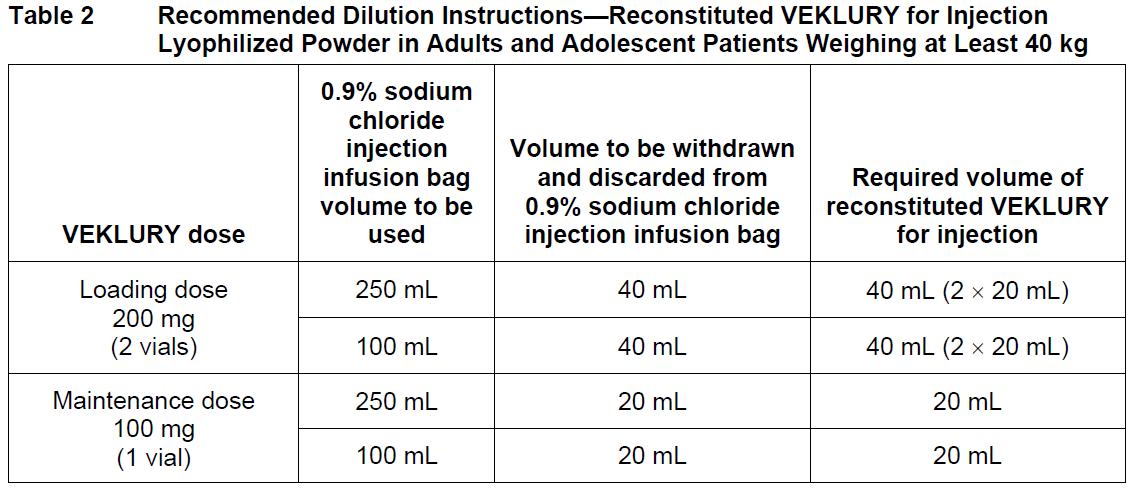 - Withdraw and discard the required volume of 0.9% sodium chloride injection from the bag following instructions in Table 1, using an appropriately sized syringe and needle. - Withdraw the required volume of reconstituted VEKLURY for injection from the VEKLURY vial following instructions in Table 1, using an appropriately sized syringe. Discard any unused portion remaining in the reconstituted vial. - Transfer the required volume of reconstituted VEKLURY for injection to the selected infusion bag. - Gently invert the bag 20 times to mix the solution in the bag. Do not shake. - The prepared infusion solution is stable for 24 hours at room temperature (20°C to 25°C) or 48 hours at refrigerated temperature (2°C to 8°C). Administration Instructions Do not administer the prepared diluted solution simultaneously with any other medication. The compatibility of VEKLURY injection with intravenous solutions and medications other than 0.9% sodium chloride injection, USP is not known. Administer VEKLURY via intravenous infusion over 30 to 120 minutes. Administration should be under conditions where management of severe hypersensitivity reactions, such as anaphylaxis, is possible. Monitor patients during infusion and observe patients for at least one hour after infusion is complete for signs and symptoms of hypersensitivity as clinically appropriate _\[see Warnings and Precautions (5.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Administer the diluted solution with the infusion rate described in Table 3. 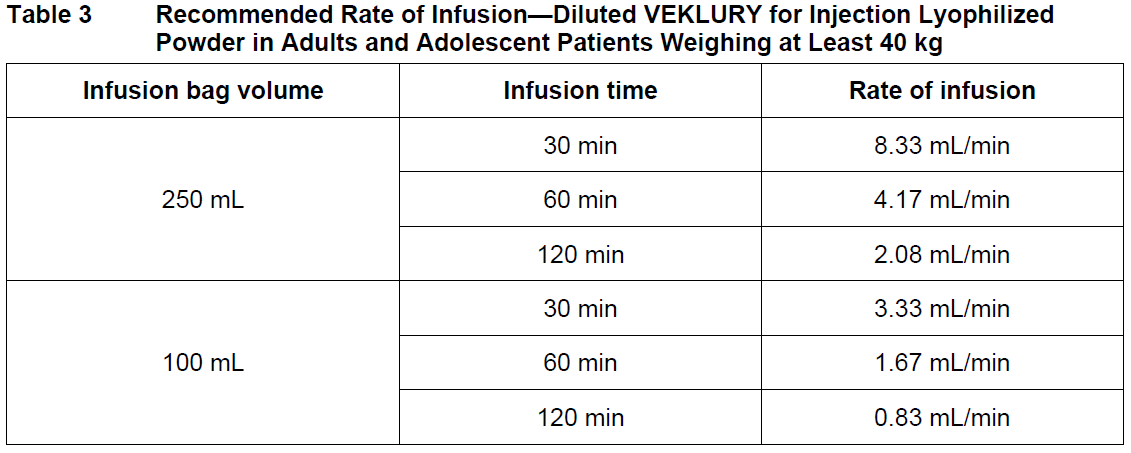 **2.7 Dosage Preparation and Administration in Pediatric Patients 28 Days of Age and Older and Weighing 3 kg to Less Than 40 kg** Reconstitution Instructions Remove the required number of single-dose vial(s) from storage. For each vial: - Aseptically reconstitute VEKLURY lyophilized powder by adding 19 mL of Sterile Water for Injection using a suitably sized syringe and needle per vial. - Only use Sterile Water for Injection to reconstitute VEKLURY lyophilized powder. - Discard the vial if a vacuum does not pull the Sterile Water for Injection into the vial. - Immediately shake the vial for 30 seconds. - Allow the contents of the vial to settle for 2 to 3 minutes. A clear, colorless to yellow solution, free of visible particles, should result. - If the contents of the vial are not completely dissolved, shake the vial again for 30 seconds and allow the contents to settle for 2 to 3 minutes. Repeat this procedure as necessary until the contents of the vial are completely dissolved. Discard the vial if the contents are not completely dissolved. - Following reconstitution, each vial contains 100 mg/20 mL (5 mg/mL) of remdesivir solution. - Use reconstituted product immediately to prepare the diluted drug product _\[see Dosage and Administration (2.7)\]_. Dilution Instructions - For pediatric patients 28 days of age and older and weighing 3 kg to less than 40 kg, the 100 mg/20 mL (5 mg/mL) remdesivir reconstituted solution should be further diluted to a fixed concentration of 1.25 mg/mL using 0.9% sodium chloride injection. - The final required infusion volume concentration of 1.25 mg/mL remdesivir diluted solution for infusion is based on the pediatric weight-based dosing regimens of 5 mg/kg for the Loading Dose and 2.5 mg/kg for each Maintenance Dose. - Small 0.9% sodium chloride injection infusion bags (e.g., 25, 50, or 100 mL) or an appropriately sized syringe should be used for pediatric dosing. The recommended dose is administered via intravenous infusion in a total volume dependent on the dose to yield the target remdesivir concentration of 1.25 mg/mL. - A syringe and syringe pump may be used for infusion volumes less than 50 mL. _Infusion with IV Bag_ - Determine the total infusion volume needed to achieve a final infusion volume concentration of 1.25 mg/mL of remdesivir diluted solution based on the patient’s calculated dose. - Select an appropriately sized infusion bag (either prefilled with 0.9% sodium chloride injection or empty) to prepare VEKLURY diluted solution. - If using a prefilled 0.9% sodium chloride injection infusion bag, withdraw and discard the amount of diluent equal to the volume of reconstituted VEKLURY solution needed per patient’s dose plus a quantity sufficient to achieve a 1.25 mg/mL final volume concentration of remdesivir diluted solution. - Withdraw the required volume of reconstituted VEKLURY solution into an appropriately sized syringe. - Transfer the required volume of reconstituted VEKLURY solution to the 0.9% sodium chloride injection infusion bag. - Gently invert the bag 20 times to mix the solution in the bag. Do not shake. - If using an empty infusion bag, transfer the required volume of reconstituted VEKLURY solution to the bag, followed by a volume of 0.9% sodium chloride injection sufficient to achieve a 1.25 mg/mL final volume concentration of remdesivir diluted solution. - The prepared infusion solution is stable for 24 hours at room temperature (20°C to 25°C) or 48 hours at refrigerated temperature (2°C to 8°C). _Infusion with Syringe_ - Determine the total infusion volume needed to achieve a final infusion volume concentration of 1.25 mg/mL of remdesivir diluted solution based on patient’s calculated dose. - Select an appropriately sized syringe equal to or larger than the calculated total infusion volume of 1.25 mg/mL remdesivir solution needed. - Withdraw the required volume of reconstituted VEKLURY solution from the vial into the syringe based on patient’s calculated dose, followed by the required volume of 0.9% sodium chloride injection needed to achieve a 1.25 mg/mL final volume concentration of remdesivir diluted solution. - Gently invert the syringe 20 times to mix the solution in the syringe. Do not shake. - The prepared diluted solution should be used immediately. Administration Instructions The prepared diluted solution should not be administered simultaneously with any other medication. The compatibility of VEKLURY with IV solutions and medications other than 0.9% sodium chloride injection, USP is not known. Administer VEKLURY via intravenous infusion over 30 to 120 minutes. The rate of infusion (mL/min) should be calculated based on the total infusion volume and total infusion time. Administration should be under conditions where management of severe hypersensitivity reactions, such as anaphylaxis, is possible. Monitor patients during infusion and observe patients for at least one hour after infusion is complete for signs and symptoms of hypersensitivity as clinically appropriate _\[see Warnings and Precautions (5.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. **2.8 Storage of Prepared Dosages** After reconstitution, use vials immediately to prepare diluted solution. The diluted VEKLURY solution in the infusion bags can be stored up to 24 hours at room temperature (20°C to 25°C) prior to administration or 48 hours at refrigerated temperature (2°C to 8°C). **IMPORTANT:** This product contains no preservative. Any unused portion of a single-dose VEKLURY vial should be discarded after a diluted solution is prepared.
INTRAVENOUS
Medical Information
**1 INDICATIONS AND USAGE** VEKLURY is indicated for the treatment of coronavirus disease 2019 (COVID-19) in: - adults and pediatric patients (28 days of age and older and weighing at least 3 kg) with pneumonia requiring supplemental oxygen. In clinical studies, there were no survival and recovery benefit with VEKLURY in patients under invasive mechanical ventilation (IMV), or under extracorporeal membrane oxygenation (ECMO). - adults and adolescent patients (aged 12 years and above and weighing at least 40 kg) who do not require supplemental oxygen and who are at high risk of progressing to severe COVID-19. _\[see Clinical Studies (13)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_
**4 CONTRAINDICATIONS** VEKLURY is contraindicated in patients with a history of clinically significant hypersensitivity reactions to VEKLURY or any components of the product _\[see Warnings and Precautions (5.1)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
J05AB16
remdesivir
Manufacturer Information
GILEAD SCIENCES SINGAPORE PTE. LTD.
Jubilant HollisterStier, LLC
Patheon Manufacturing Services LLC
Hikma Farmaceutica (Portugal) S.A.
Hospira, Inc.
Patheon Italia S.p.A.
Valdepharm
Xellia Pharmaceuticals USA LLC
Active Ingredients
Documents
Package Inserts
Veklury Package Insert.pdf
Approved: March 30, 2023
