Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
POWDER, FOR SUSPENSION
**Recommended Dosage** Oral azithromycin should be administered as a single daily dose. The period of dosing with regard to infection is given below. Azithromycin tablets and powder for oral suspension can be taken with or without food. For the treatment of sexually transmitted diseases caused by Chlamydia trachomatis, Haemophilus ducreyi, or susceptible Neisseria gonorrhoea, the dose is 1000 mg as a single oral dose. For prophylaxis against MAC infections in patients infected with the human immunodeficiency virus (HIV), the dose is 1200 mg once per week. For all other indications in which the oral formulation is administered, the total dosage of 1500 mg should be given as 500 mg daily for 3 days. As an alternative, the same total dose can be given over 5 days with 500 mg given on day 1, then 250 mg daily on days 2 to 5. Children The maximum recommended total dose for any treatment is 1500 mg for children. In general, the total dose in children is 30 mg/kg. The total dose of 30 mg/kg should be given as a single daily dose of 10 mg/kg daily for 3 days, or given over 5 days with a single daily dose of 10 mg/kg on day 1, then 5 mg/kg on days 2–5. For children weighing less than 15 kg, azithromycin suspension should be measured as closely as possible. For children weighing 15 kg or more, azithromycin suspension should be administered according to the guide provided below: 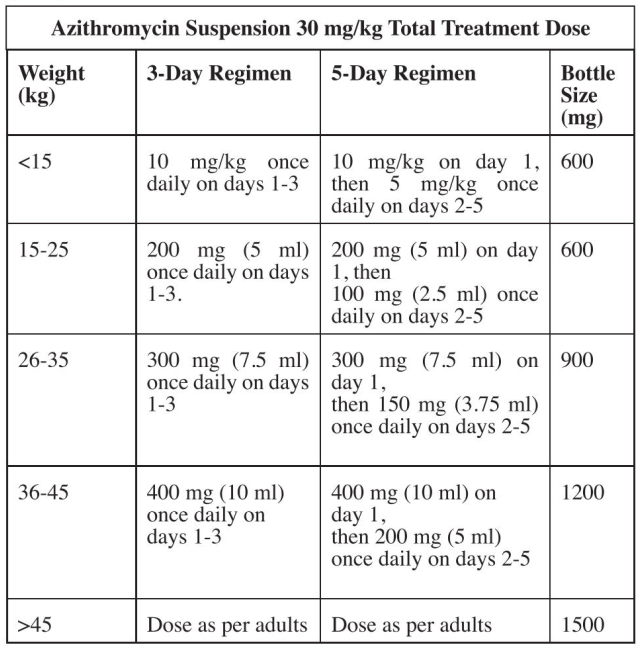 Azithromycin tablets should only be administered to children weighing more than 45 kg. Safety and efficacy for the prevention or treatment of MAC in children have not been established. Based on pediatric pharmacokinetic data, a dose of 20 mg/kg would be similar to the adult dose of 1200 mg but with a higher Cmax. **Elderly** The same dosage as in adult patients is used in the elderly. **Patients with Renal Impairment** No dose adjustment is necessary in patients with mild to moderate renal impairment (GFR 10 – 80 ml/min). Caution should be exercised when azithromycin is administered to patients with severe renal impairment (GFR < 10 ml/min). **Patients with Hepatic Impairment** The same dosage as in patients with normal hepatic function may be used in patients with mild to moderate hepatic impairment. **Mode of Administration** Oral
ORAL
Medical Information
**Indications** Azithromycin is indicated for infections caused by susceptible organisms; in lower respiratory tract infections including bronchitis and pneumonia, in skin and soft tissue infections, in acute otitis media and in upper respiratory tract infections including sinusitis and pharyngitis/tonsillitis. (Penicillin is the usual drug of choice in the treatment of Streptococcus pyogenes pharyngitis, including the prophylaxis of rheumatic fever. Azithromycin is generally effective in the eradication of streptococci from the oropharynx, however, data establishing the efficacy of azithromycin and the subsequent prevention of rheumatic fever are not available at present.) In sexually transmitted diseases in men and women, azithromycin is indicated in the treatment of uncomplicated genital infections due to Chlamydia trachomatis. It is also indicated in the treatment of chancroid due to Haemophilus ducreyi; and uncomplicated genital infection due to non-multiresistant Neisseria gonorrhoea; concurrent infection with Treponema pallidum should be excluded. Azithromycin is indicated, either alone or in combination with rifabutin, for prophylaxis against Mycobacterium avium-intracellulare complex (MAC) infection, an opportunistic infection prevalent in patients with advanced human immunodeficiency virus (HIV).
**Contraindications** The use of this product is contraindicated in patients with hypersensitivity to azithromycin, erythromycin, any macrolide or ketoIide antibiotic.
J01FA10
azithromycin
Manufacturer Information
ZIWELL MEDICAL (S) PTE LTD
Laboratorios Lesvi,S.L
