Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
SUSPENSION
**DOSAGE AND ADMINISTRATION:** **Important Administration Instructions for NOXAFIL Delayed Release Tablet and Oral Suspension** **Non-Interchangeability between NOXAFIL Delayed Release Tablets and NOXAFIL Oral Suspension** The prescriber should follow the specific dosing instructions for each formulation. The tablet and oral suspension are not to be used interchangeably due to the differences in the dosing of each formulation. **NOXAFIL Delayed Release Tablets** NOXAFIL Delayed Release Tablets may be taken without regard to food intake. NOXAFIL Delayed Release Tablets should be swallowed whole, and not be divided, crushed, or chewed. 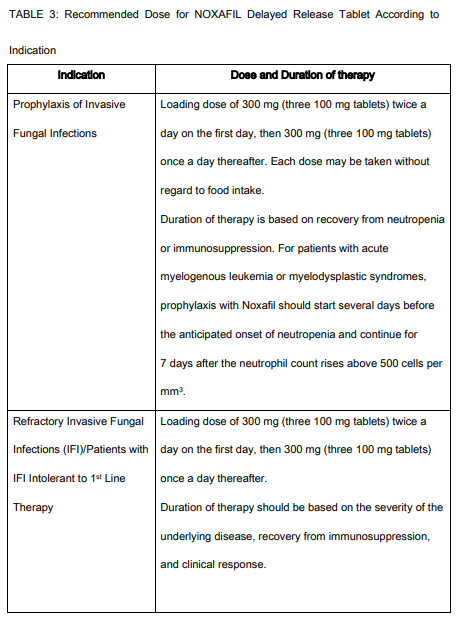 **NOXAFIL Oral Suspension** NOXAFIL Oral Suspension should be administered with a meal, or with 240 ml of a nutritional supplement to enhance the oral absorption and to ensure adequate exposure. The oral suspension must be shaken well before use. 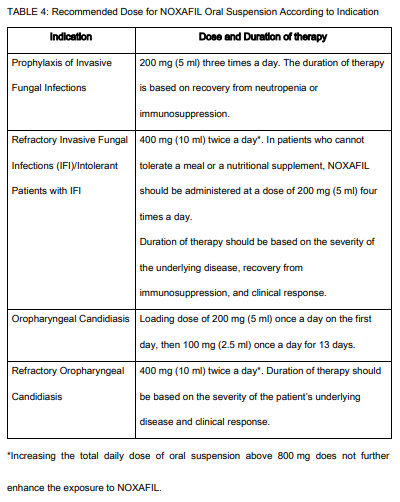 **Use in renal impairment:** No dose adjustment is required for renal dysfunction and NOXAFIL is not significantly renally eliminated, an effect of severe renal insufficiency on the pharmacokinetics of NOXAFIL is not expected and no dose adjustment is recommended. **Use in hepatic impairment:** There are limited pharmacokinetic data in patients with hepatic insufficiency; therefore, no recommendation for dose adjustment can be made. In the small number of subjects studied who had hepatic insufficiency, there was an increase in half-life with a decrease in hepatic function. **Use in children:** Safety and efficacy in children below the age of 13 years have not been established.
ORAL
Medical Information
**INDICATIONS AND USAGE:** NOXAFIL Delayed Release Tablets and Oral Suspension are indicated for prophylaxis of invasive _Aspergillus_ and _Candida_ infections, including both yeasts and molds, in patients, 13 years of age and older, who are at high risk of developing these infections, such as patients with prolonged neutropenia or hematopoietic stem cell transplant (HSCT) recipients. NOXAFIL Delayed Release Tablets and Oral Suspension are indicated for use in the treatment of the following fungal infections in patients 13 years of age or older: - Refractory Invasive Fungal infections/Intolerant Patients with IFI: Fusariosis, zygomycosis, cryptococcosis, coccidioidomycosis, chromoblastomycosis, and mycetoma in patients with disease refractory to other therapy, or patients who are intolerant of other therapy. Refractoriness is defined as progression of infection or failure to improve after a minimum of 7 days of prior therapeutic doses of effective antifungal therapy. NOXAFIL Oral Suspension is also indicated for use in the treatment of the following fungal infections in patients 13 years of age or older: - Oropharyngeal candidiasis in patients who have severe disease or who are immunocompromised, including in patients with disease that is refractory to itraconazole and fluconazole. Refractoriness is defined as progression of infection or failure to improve after a minimum of 7 days of prior therapeutic doses of effective antifungal therapy.
**CONTRAINDICATIONS:** NOXAFIL is contraindicated in patients with known hypersensitivity to NOXAFIL or any component of the product. Although not studied _in vitro_ or _in vivo_, coadministration of the CYP3A4 substrates terfenadine, astemizole, cisapride, pimozide, or quinidine with NOXAFIL are contraindicated since increased plasma concentrations of these drugs can lead to QT prolongation and rare occurrences of torsade de pointes. Coadministration with the HMG-CoA reductase inhibitors that are primarily metabolized through CYP3A4 is contraindicated since increased plasma concentration of these drugs can lead to rhabdomyolysis. Although not studied _in vitro_ or _in vivo_, NOXAFIL may increase the plasma concentrations of ergot alkaloids which may lead to ergotism. Coadministration of NOXAFIL and ergot alkaloids is contraindicated.
J02AC04
posaconazole
Manufacturer Information
MSD PHARMA (SINGAPORE) PTE. LTD.
Patheon Inc.
Active Ingredients
Documents
Package Inserts
Noxafil PI.pdf
Approved: January 12, 2023
