Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
GENERATOR
**4.2. Posology and method of administration** This medicinal product is for use in designated nuclear medicine facilities only, and should only be handled by authorised personnel. Posology Sodium pertechnetate (99mTc) is normally administered intravenously at activities which vary widely according to the clinical information required and the equipment employed. Other activities may be justifiable. The injection of activities greater than local DRLs (Diagnostic Reference Levels) should be justified. Pre-treatment of patients with thyroid blocking agents or reducing agents may be necessary for certain indications. Recommended activities are as follows: _Adults (70 kg) and elderly population:_ Thyroid scintigraphy: 20–80 MBq Salivary gland scintigraphy: 30 to 150 MBq for static images up to 370 MBq for dynamic images. Meckel’s diverticulum scintigraphy: 300–400 MBq Lacrimal duct scintigraphy: 2–4 MBq each eye. Renal impairment Careful consideration of the activity to be administered is required since an increased radiation exposure is possible in these patients. Paediatric population The use in children and adolescents has to be considered carefully, based upon clinical needs and assessing the risk/benefit ratio in this patient group. The activities to be administered to children and to adolescents may be calculated according to the European Association of Nuclear Medicine (EANM-May 2008) guidelines, by using the formula corresponding to the indication concerned and the relevant correction factor corresponding to the body mass of the young patient (see Table 1). Thyroid scintigraphy: Activity administered \[MBq\] = 5.6 MBq x Correction factor (Table 1), With a minimal activity of 10 MBq necessary for obtaining images of satisfactory quality. Identification of ectopic gastric mucosa: Activity administered \[MBq\] = 10.5 MBq x Correction factor (Table 1), With a minimal activity of 20 MBq necessary for obtaining images of satisfactory quality. 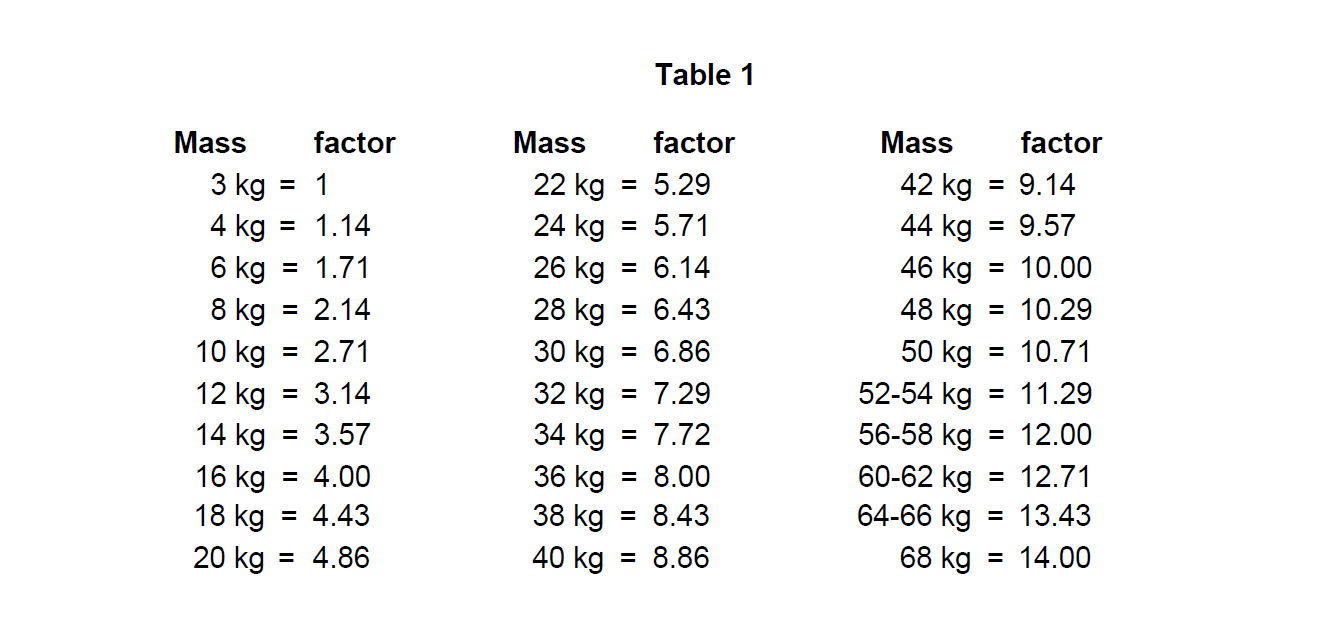 Salivary gland scintigraphy: The Paediatric Task Group of EANM (1990) recommends that the activity to be administered to a child should be calculated from the body weight according to the following table 2: 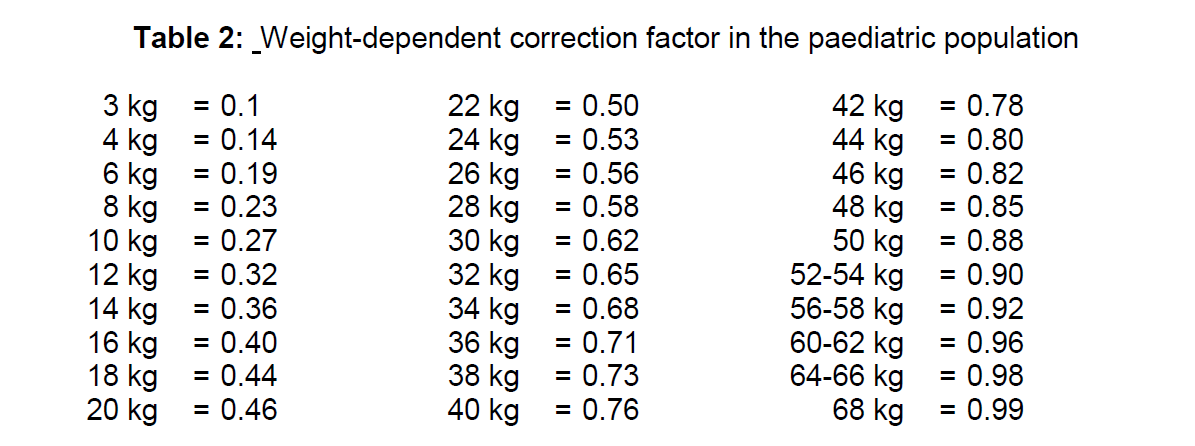 In very young children, a minimum dose of 10 MBq is necessary in order to obtain images of sufficient quality. Lacrimal duct scintigraphy: Recommended activities for adults apply for children. Method of administration For multidose use. For intravenous or ocular use or labeling. For instructions on extemporary preparation of the medicinal product before administration, see section 12 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. For patient preparation, see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. In thyroid scintigraphy, salivary gland scintigraphy and identification of ectopic gastric mucosa, the sodium pertechnetate (99mTc) solution is administered by intravenous injection. In lacrimal duct scintigraphy, drops are instilled in each eye. Image acquisition Thyroid scintigraphy: 20 minutes after intravenous injection. Salivary gland scintigraphy: Immediately after intravenous injection and at regular intervals up to 15 minutes. Identification of ectopic gastric mucosa: immediately after intravenous injection and at regular intervals for 30 minutes. Lacrimal duct scintigraphy: dynamic acquisition within 2 minutes after instillation, followed by static images acquired at regular intervals within 20 minutes.
INTRAVENOUS
Medical Information
**4.1. Therapeutic indications** This medicinal product is for diagnostic use only. The eluate from the radionuclide generator (Sodium Pertechnetate (99mTc) Injection Ph. Eur.) is indicated for: - Labelling of various kits for radiopharmaceutical preparation developed and approved for radiolabelling with such solution. - Thyroid scintigraphy: direct imaging and measurement of thyroid uptake to give information on the size, position, nodularity and function of the gland in thyroid disease. - Salivary gland scintigraphy: diagnosis of chronic sialadenitis (e.g. Sjögren Syndrom) as well as to assess salivary gland function and duct patency in salivary glands disorders and monitoring of the response to therapeutic interventions (in particular radio iodine therapy). - Location of ectopic gastric mucosa (Meckel’s diverticulum). - Lacrimal duct scintigraphy to assess functional disorders of lacrimation and monitoring of the response to therapeutic interventions.
**4.3. Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
V09
诊断用放射性药物
Manufacturer Information
QT INSTRUMENTS (S) PTE LTD
CIS bio International
Laboratoires Chaix Et Du Marais (Flushing Solution)
Laboratoire Bioluz (Elution Solution)
Active Ingredients
Documents
Package Inserts
TEKCIS PI.pdf
Approved: July 10, 2019
