Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** Accofil therapy should only be given in collaboration with an oncology centre which has experience in granulocyte-colony stimulating factor (G-CSF) treatment and haematology and has the necessary diagnostic facilities. The mobilisation and apheresis procedures should be performed in collaboration with an oncology-haematology centre with acceptable experience in this field and where the monitoring of haematopoietic progenitor cells can be correctly performed. Posology _Established cytotoxic chemotherapy_ The recommended dose of filgrastim is 0.5 million units/kg/day (5 micrograms/kg/day). The first dose of Accofil should not be administered less than 24 hours following cytotoxic chemotherapy. In randomised clinical trials, a subcutaneous dose of 230 microgram/m2/day (4.0 to 8.4 microgram/kg/day) was used. Daily dosing with filgrastim should continue until the expected neutrophil nadir is passed and the neutrophil count has recovered to the normal range. Following established chemotherapy for solid tumours, lymphomas, and lymphoid leukaemias, it is expected that the duration of treatment required to fulfil these criteria will be up to 14 days. Following induction and consolidation treatment for acute myeloid leukaemia the duration of treatment may be substantially longer (up to 38 days) depending on the type, dose and schedule of cytotoxic chemotherapy used. In patients receiving cytotoxic chemotherapy, a transient increase in neutrophil counts is typically seen 1–2 days after initiation of filgrastim therapy. However, for a sustained therapeutic response, filgrastim therapy should not be discontinued before the expected nadir has passed and the neutrophil count has recovered to the normal range. Premature discontinuation of filgrastim therapy, prior to the time of the expected neutrophil nadir, is not recommended. _In patients treated with myeloablative therapy followed by bone marrow transplantation_ The recommended starting dose of filgrastim is 1.0 million units/kg/day (10 micrograms/kg/day). The first dose of filgrastim should be administered at least 24 hours after cytotoxic chemotherapy and at least 24 hours after bone marrow infusion. The efficacy and safety of filgrastim given for longer than 28 days in this setting have not been established. Once the neutrophil nadir has been passed, the daily dose of filgrastim should be titrated against the neutrophil response as follows: 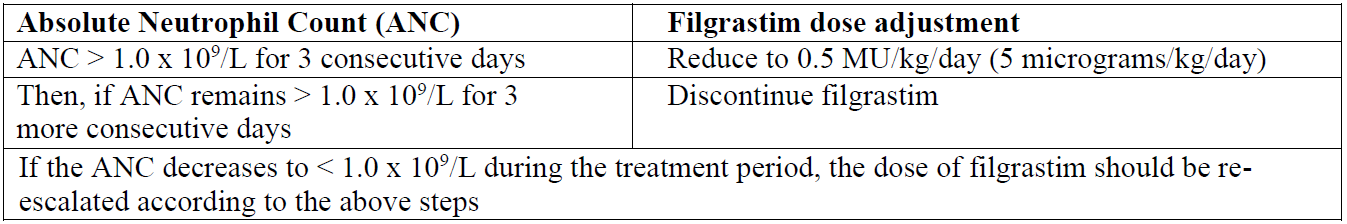 _Mobilisation of peripheral blood progenitor cells (PBPC)_ _Mobilisation of Peripheral Blood Progenitor Cells (PBPC) in patients undergoing myelosuppressive or myeloablative therapy followed by autologous peripheral blood progenitor cell transplantation with or without bone marrow transplantation_ The recommended dose of filgrastim for PBPC mobilisation when used alone is 1.0 million units/kg/day (10 micrograms/kg/day) as a 24-hour subcutaneous continuous infusion or a single daily subcutaneous injection for 5–7 consecutive days. The timing of leukapheresis: 1 or 2 leukaphereses on days 5 and 6 which is often sufficient. In other circumstances, additional leukaphereses may be necessary. Filgrastim dosing should be maintained until the last leukapheresis. The recommended dose of filgrastim for PBPC mobilisation after myelosuppressive chemotherapy is 0.5 million units/kg/day (5 micrograms/kg/day) given daily by subcutaneous injection from the first day after completion of chemotherapy until the expected neutrophil nadir is passed and the neutrophil count has recovered to the normal range. Leukapheresis should be performed during the period when the ANC rises from < 0.5 x 109/L to > 5.0 x 109/L. For patients who have not had extensive chemotherapy, one leukapheresis is often sufficient. In other circumstances, additional leukaphereses are recommended. _Mobilisation of Peripheral Blood Progenitor Cells (PBPC) in normal donors prior to allogeneic peripheral blood progenitor cell transplantation_ For PBPC mobilisation in normal donors, filgrastim should be administered at 1.0 million units kg/day (10 micrograms/kg/day) subcutaneously for 4 – 5 consecutive days. Leukapheresis should be started at day 5 and continued until day 6 if needed in order to collect 4 x 106 CD34+ cells/kg recipient body weight. _Severe chronic neutropenia (SCN)_ _Congenital neutropenia_ The recommended starting dose is 1.2 million units/kg/day (12 micrograms/kg/day) subcutaneously as a single dose or in divided doses. _Idiopathic or cyclic neutropenia_ The recommended starting dose is 0.5 million units/kg/day (5 micrograms/kg/day) subcutaneously as a single dose or in divided doses. _Dose adjustments:_ Filgrastim should be administered daily by subcutaneous injection until the neutrophil count has reached and can be maintained at more than 1.5 x 109/L. When the response has been obtained, the minimal effective dose to maintain this level should be established. Long-term daily administration is required to maintain an adequate neutrophil count. After one to two weeks of therapy, the initial dose may be doubled or halved depending upon the patient’s response. Subsequently, the dose may be individually adjusted every 1–2 weeks to maintain the average neutrophil count between 1.5 x 109/L and 10 x 109/L. A faster schedule of dose escalation may be considered in patients presenting with severe infections. In clinical studies, 97% of patients who responded had a complete response at doses of ≤ 2.4 million units/kg/day (24 micrograms/kg/day). The long-term safety of administration of filgrastim at doses above 2.4 million units/kg/day (24 micrograms/kg/day) in patients with SCN has not been established. _HIV infection_ _For reversal of neutropenia_ The recommended starting dose of filgrastim is 0.1 million units/kg/day (1 micrograms/kg/day) given daily by subcutaneous injection with titration up to a maximum of 0.4 million units/kg/day (4 micrograms/kg/day) until a normal neutrophil count is reached and can be maintained (ANC > 2.0 x 109/L). In clinical studies, more than 90% of patients responded at these doses, achieving a reversal of neutropenia in a median of 2 days. In a small number of patients (< 10%), doses up to 1.0 million units/kg/day (10 micrograms/kg/day) were required to achieve reversal of neutropenia. _For maintenance of normal neutrophil counts_ When reversal of neutropenia has been achieved, the minimal effective dose to maintain a normal neutrophil count should be established. Initial dose adjustment to alternate day dosing with 30 million units/day (300 micrograms/day) by subcutaneous injection is recommended. Further dose adjustment may be necessary, as determined by the patient’s ANC, to maintain the neutrophil count at > 2.0 x 109/L. In clinical studies, dosing with 30 million units/day (300 micrograms/day) on 1 – 7 days per week was required to maintain the ANC > 2.0 x 109/L, with the median dose frequency being 3 days per week. Long-term administration may be required to maintain the ANC > 2.0 x 109/L. _For Maintaining Normal Neutrophil Counts:_ When reversal of neutropenia has been achieved, the minimal effective dose to maintain a normal neutrophil count should be established. Initial dose adjustment to alternate day dosing with 30 million units (300 micrograms)/day by subcutaneous injection is recommended. Further dose adjustment may be necessary, as determined by the patient’s ANC, to maintain the neutrophil count at > 2.0 x 109/L. In clinical studies, dosing with 30 million units (300 micrograms)/day on 1 to 7 days per week was required to maintain the ANC > 2.0 x 109/L, with the median dose frequency being 3 days per week. Long-term administration may be required to maintain the ANC > 2.0 x 109/L. _Special populations_ _Elderly patients_ Clinical trials with filgrastim have included a small number of elderly patients but special studies have not been performed in this group and therefore specific posology recommendations cannot be made. _Patients with renal/hepatic impairment_ Studies of filgrastim in patients with severe impairment of renal or hepatic function demonstrate that it exhibits a similar pharmacokinetic and pharmacodynamic profile to that seen in normal individuals. Dose adjustment is not required in these circumstances. _Paediatric patients in established cytotoxic chemotherapy_ The safety and efficacy of filgrastim are similar in adults and children receiving cytotoxic chemotherapy. _In patients undergoing myelosuppressive or myeloablative therapy followed by autologous peripheral blood progenitor cell transplantation_ The safety and efficacy of filgrastim have not been assessed in normal donors < 16 years. _Paediatric patients with severe chronic neutropenia (SCN)_ The safety and efficacy in neonates have not been established. Long term administration of filgrastim is indicated in children with severe congenital, cyclic or idiopathic neutropenia with an Absolute Neutrophil Count (ANC) ≤ 0.5 x 109/L, and a history of severe or recurrent infections, to increase neutrophil counts and to reduce the incidence and duration of infection-related events. _Paediatric patients in the SCN and cancer settings_ Sixty-five percent of patients studied in a SCN trial program with filgrastim administration, were under 18 years of age. The efficacy of the treatment was clear for this age group, which included most patients with congenital neutropenia. There were no differences in the safety profiles for paediatric patients treated for SCN. Method of administration _Established cytotoxic chemotherapy_ Filgrastim may be administered as a daily subcutaneous injection or alternatively as a daily intravenous infusion diluted in glucose 50 mg/ml (5%) solution over 30 minutes. For further instructions on dilution prior to infusion see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The subcutaneous route is preferred in most cases. There is some evidence from a study of single dose administration that intravenous dosing may shorten the duration of effect. The clinical relevance of this finding to multiple dose administration is not clear. The choice of route should depend on the individual clinical circumstance. In randomised clinical studies, a subcutaneous dose of 23 million units/m2/day (230 micrograms/m2/day) or rather 4–8.4 micrograms/kg/day was used. _Patients treated with myeloablative therapy followed by bone marrow transplantation_ Filgrastim is administered as an intravenous short-term infusion over 30 minutes or as a subcutaneous or intravenous continuous infusion over 24 hours, in each case after dilution in 20 ml of glucose 50 mg/ml (5%) solution. For further instructions on dilution with glucose 50 mg/ml (5%) solution prior to infusion see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. _In patients with Mobilisation of PBPC_ Filgrastim for PBPC mobilisation when used alone: Filgrastim may be given as a 24-hour subcutaneous continuous infusion or subcutaneous injection. For infusions filgrastim should be diluted in 20ml of 5% glucose solution (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Filgrastim for PBPC mobilisation after myelosuppressive chemotherapy: Filgrastim should be given by subcutaneous injection. _For the mobilisation of PBPCs in normal donors prior to allogeneic PBPC transplantation_ Filgrastim should be given by subcutaneous injection. _In patients with SCN_ Congenital, idiopathic or cyclic neutropenia; filgrastim should be given by subcutaneous injection. _In patients with HIV infection_ For the reversal of neutropenia and maintenance of normal neutrophil counts in patients with HIV infection, filgrastim is administered subcutaneously.
INTRAVENOUS, SUBCUTANEOUS
Medical Information
**4.1 Therapeutic indications** _Established cytotoxic chemotherapy_ Accofil is indicated for the reduction in the duration of neutropenia and the incidence of febrile neutropenia in patients treated with established cytotoxic chemotherapy for malignancy (with the exception of chronic myeloid leukaemia and myelodysplastic syndromes) and for the reduction in the duration of neutropenia and its clinical sequelae in patients undergoing myeloablative therapy followed by bone marrow transplantation considered to be at increased risk of prolonged severe neutropenia. _Peripheral blood progenitor cell mobilisation (PBPC)_ Accofil is indicated for the mobilisation of autologous peripheral blood progenitor cells (PBPC) alone, or following myelosuppressive chemotherapy and the mobilisation of peripheral blood progenitor cells in normal donors (allogeneic PBPC). _Severe chronic neutropenia (SCN)_ Long-term administration of Neupogen is indicated in patients, children or adults, with severe congenital, cyclic or idiopathic neutropenia with an Absolute Neutrophil Count (ANC) ≤ 0.5 x 109/l, and a history of severe or recurrent infections, to increase neutrophil counts and to reduce the incidence and duration of infection-related events. _HIV infection_ Accofil is indicated for the treatment of persistent neutropenia (ANC ≤ 1.0 x 109/L) in patients with advanced HIV infection, in order to reduce the risk of bacterial infections when other options to manage neutropenia are inappropriate.
**4.3 Contraindications** Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
L03AA02
filgrastim
Manufacturer Information
ACCORD HEALTHCARE PRIVATE LIMITED
Intas Pharmaceuticals Limited Biopharma Division
Active Ingredients
Documents
Package Inserts
ACCOFIL PI.pdf
Approved: July 8, 2021
