Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**Dosage and Administration** DARZALEX® SC is for subcutaneous use only. DARZALEX® SC has different dosage and administration instructions than intravenous daratumumab. Do not administer intravenously. For patients currently receiving daratumumab intravenous formulation, DARZALEX® SC may be used as an alternative to the intravenous daratumumab formulation starting at the next scheduled dose. DARZALEX® SC should be administered by a healthcare professional, and the first dose should be administered in an environment where resuscitation facilities are available. Pre- and post-injection medications should be administered (see _Recommended concomitant medications_ below). **Dosage – Adults (≥18 years)** _**Recommended dose for multiple myeloma**_ The DARZALEX® SC dosing schedule in Table 1 is for combination therapy with 4-week cycle regimens (e.g. lenalidomide, pomalidomide, carfilzomib) and for monotherapy as follows: - combination therapy with lenalidomide and low-dose dexamethasone for patients with newly diagnosed multiple myeloma ineligible for autologous stem cell transplant (ASCT) - combination therapy with lenalidomide or pomalidomide and low-dose dexamethasone for patients with relapsed/refractory multiple myeloma - combination therapy with carfilzomib and low-dose dexamethasone for patients with relapsed/refractory multiple myeloma - monotherapy for patients with relapsed/refractory multiple myeloma The recommended dose is DARZALEX® SC 1800 mg administered subcutaneously, over approximately 3–5 minutes, according to the following dosing schedule: 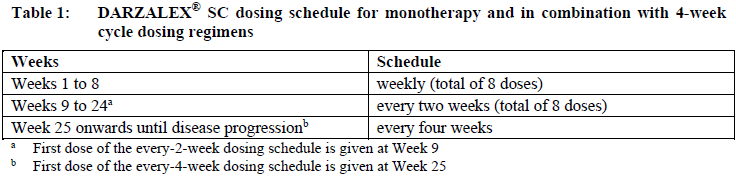 For dosing instructions of medicinal products administered with DARZALEX® SC, see _Clinical Studies_ and manufacturer’s prescribing information – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The DARZALEX® SC dosing schedule in Table 2 is for combination therapy with bortezomib, melphalan and prednisone (6-week cycle regimen) for patients with newly diagnosed multiple myeloma ineligible for ASCT. The recommended dose is DARZALEX® SC 1800 mg administered subcutaneously, over approximately 3–5 minutes, according to the following dosing schedule: 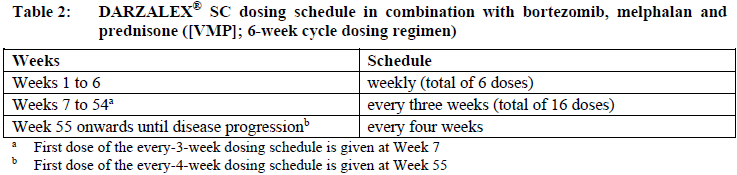 Bortezomib is given twice weekly at Weeks 1, 2, 4 and 5 for the first 6-week cycle, followed by once weekly at Weeks 1, 2, 4 and 5 for eight more 6-week cycles. For information on the VMP dose and dosing schedule when administered with DARZALEX® SC, see _Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The DARZALEX® SC dosing schedule in Table 3 is for combination therapy with bortezomib, thalidomide and dexamethasone (4-week cycle regimens) for treatment of newly diagnosed multiple myeloma patients eligible for ASCT. The recommended dose is DARZALEX® SC 1800 mg administered subcutaneously, over approximately 3–5 minutes, according to the following dosing schedule: 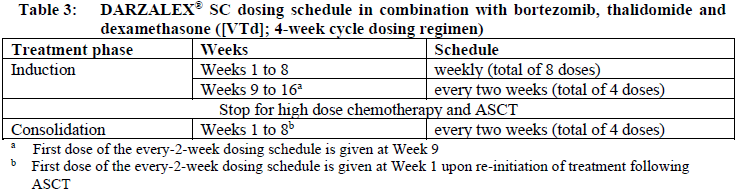 For dosing instructions of medicinal products administered with DARZALEX® SC, see _Clinical Studies_ and manufacturer’s prescribing information – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The DARZALEX® SC dosing schedule in Table 4 is for combination therapy with 3-week cycle regimens (e.g. bortezomib) for patients with relapsed/refractory multiple myeloma. The recommended dose is DARZALEX® SC 1800 mg administered subcutaneously, over approximately 3–5 minutes, according to the following dosing schedule: 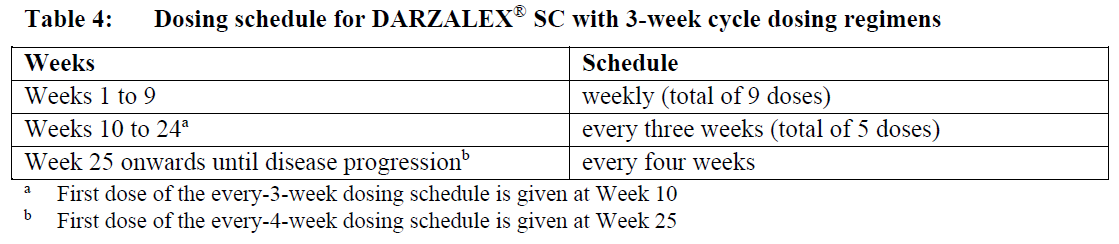 For dosing instructions for medicinal products administered with DARZALEX® SC see _Clinical Studies_ and manufacturer’s prescribing information – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. _**Recommended dose for AL amyloidosis**_ The DARZALEX® SC dosing schedule in Table 5 is for combination therapy with bortezomib, cyclophosphamide and dexamethasone (4-week cycle regimen) for patients with AL amyloidosis. The recommended dose is DARZALEX® 1800 mg administered subcutaneously, over approximately 3–5 minutes, according to the following dosing schedule: 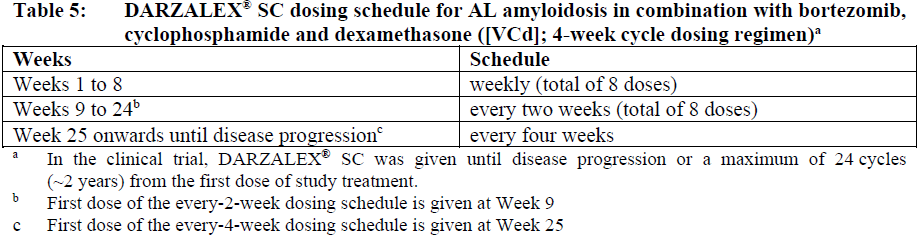 For dosing instructions of medicinal products administered with DARZALEX® SC, see _Clinical Studies_ and manufacturer’s prescribing information – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. _**Missed dose (s)**_ If a planned dose of DARZALEX® SC is missed, administer the dose as soon as possible and adjust the dosing schedule accordingly, maintaining the treatment interval. _**Dose modifications**_ No dose reductions of DARZALEX® SC are recommended. Dose delay may be required to allow recovery of blood cell counts in the event of hematological toxicity (see _Warnings and Precautions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For information concerning medicinal products given in combination with DARZALEX® SC, see manufacturer’s prescribing information. DARZALEX® SC and management of infusion-related reactions: In clinical trials, no modification to rate or dose of DARZALEX® SC was required to manage infusion-related reactions. **_Recommended concomitant medications_** _Pre-injection medication_ Pre-injection medications (oral or intravenous) should be administered to reduce the risk of infusion-related reactions (IRRs) to all patients 1–3 hours prior to every administration of DARZALEX® subcutaneous injection as follows: - Corticosteroid (long-acting or intermediate-acting) Monotherapy: Methylprednisolone 100 mg, or equivalent. Following the second injection, the dose of corticosteroid may be reduced to methylprednisolone 60 mg. Combination therapy: Administer 20 mg dexamethasone (or equivalent) prior to every DARZALEX® SC injection. When dexamethasone is the background-regimen specific corticosteroid, the dexamethasone treatment dose will instead serve as pre-medication on DARZALEX® SC administration days (see _Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Additional background-regimen specific corticosteroids (e.g. prednisone) should not be taken on DARZALEX® SC administration days when patients have received dexamethasone (or equivalent) as a pre-medication. - Antipyretics (paracetamol/acetaminophen 650 to 1000 mg) - Antihistamine (diphenhydramine 25 to 50 mg or equivalent) _Post-injection medication_ Administer post-injection medication to reduce the risk of delayed IRRs as follows: - Monotherapy: Administer oral corticosteroid (20 mg methylprednisolone or equivalent dose of an intermediate-acting or long-acting corticosteroid in accordance with local standards) on each of the 2 days following all DARZALEX® SC injections (beginning the day after the injection). - Combination therapy: Consider administering low-dose oral methylprednisolone (≤ 20 mg) or equivalent the day after the DARZALEX® SC injection. However, if a background regimen-specific corticosteroid (e.g. dexamethasone, prednisone) is administered the day after the DARZALEX® SC injection, additional post-injection medications may not be needed (see _Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). If the patient experiences no major IRRs after the first three injections, post-injection corticosteroids (excluding any background regimen corticosteroids) may be discontinued. Additionally, for patients with a history of chronic obstructive pulmonary disease, consider the use of post-injection medications including short and long acting bronchodilators, and inhaled corticosteroids. Following the first four injections, if the patient experiences no major IRRs, these inhaled post-injection medications may be discontinued at the discretion of the physician. _Prophylaxis for herpes zoster virus reactivation_ Anti-viral prophylaxis should be considered for the prevention of herpes zoster virus reactivation. If required, anti-viral prophylaxis is recommended to be initiated within 1 week of starting DARZALEX® SC. **Special populations** _**Pediatrics (17 years of age and younger)**_ The safety and efficacy of DARZALEX® SC have not been established in pediatric patients. _**Elderly (65 years of age and older)**_ No dose adjustments are considered necessary in elderly patients (see _Pharmacokinetic Properties, Adverse Reactions_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Renal impairment**_ No formal studies of daratumumab in patients with renal impairment have been conducted. Based on population pharmacokinetic (PK) analyses, no dosage adjustment is necessary for patients with renal impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Hepatic impairment**_ No formal studies of daratumumab in patients with hepatic impairment have been conducted. Changes in hepatic function are unlikely to have any effect on the elimination of daratumumab since IgG1 molecules such as daratumumab are not metabolized through hepatic pathways. No dosage adjustments are necessary for patients with hepatic impairment (see _Pharmacokinetic Properties_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Cardiac disease**_ AL amyloidosis patients with advanced cardiac disease (Mayo Stage IIIB or NYHA Class IIIB or IV) have not been studied in clinical trials with DARZALEX® SC. **Administration** DARZALEX® SC should be administered by a healthcare professional. To prevent medication errors, it is important to check the vial labels to ensure that the drug being prepared and administered is DARZALEX® for subcutaneous injection and not intravenous daratumumab. DARZALEX® subcutaneous (SC) formulation is not intended for intravenous administration and should be administered via a subcutaneous injection only. DARZALEX® SC is for single use only and is ready to use. - DARZALEX® SC is compatible with polypropylene or polyethylene syringe material; polypropylene, polyethylene, or polyvinyl chloride (PVC) subcutaneous infusion sets; and stainless steel transfer and injection needles. - DARZALEX® SC should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if opaque particles, discoloration or other foreign particles are present. - Remove the DARZALEX® SC vial from refrigerated storage \[2°C–8°C (36°F–46°F)\] and equilibrate to ambient temperature \[15°C–30°C (59°F–86°F)\]. The unpunctured vial may be stored at ambient temperature and ambient light for a maximum of 24 hours. Keep out of direct sunlight. Do not shake. - Prepare the dosing syringe in controlled and validated aseptic conditions. - To avoid needle clogging, attach the hypodermic injection needle or subcutaneous infusion set to the syringe immediately prior to injection. Storage of prepared syringe - If the syringe containing DARZALEX® SC is not used immediately, store the DARZALEX® SC solution for up to 24 hours refrigerated followed by up to 7 hours at 15°C–30°C (59°F–86°F) and ambient light. Discard if stored more than 24 hours of being refrigerated or more than 7 hours of being at 15°C–30°C (59°F–86°F), if not used. If stored in the refrigerator, allow the solution to come to ambient temperature before administration. Administration - Inject 15 mL DARZALEX® SC into the subcutaneous tissue of the abdomen approximately 3 inches \[7.5 cm\] to the right or left of the navel over approximately 3–5 minutes. Do not inject DARZALEX® SC at other sites of the body as no data are available. - Injection sites should be rotated for successive injections. - DARZALEX® SC should never be injected into areas where the skin is red, bruised, tender, hard or areas where there are scars. - Pause or slow down delivery rate if the patient experiences pain. In the event pain is not alleviated by slowing down the injection, a second injection site may be chosen on the opposite side of the abdomen to deliver the remainder of the dose. - During treatment with DARZALEX® SC, do not administer other medications for subcutaneous use at the same site as DARZALEX® SC. - Any waste material should be disposed in accordance with local requirements.
SUBCUTANEOUS
Medical Information
**Indications** DARZALEX® SC is indicated for the treatment of patients with multiple myeloma: - in combination with bortezomib, melphalan and prednisone, or in combination with lenalidomide and dexamethasone in newly diagnosed patients who are ineligible for autologous stem cell transplant. - in combination with bortezomib, thalidomide, and dexamethasone in newly diagnosed patients who are eligible for autologous stem cell transplant. - in combination with lenalidomide and dexamethasone, or in combination with bortezomib and dexamethasone in patients who have received at least one prior therapy. - in combination with pomalidomide and dexamethasone in patients who have received one prior therapy including lenalidomide and a proteasome inhibitor (PI) and were lenalidomide-refractory, or who have received at least two prior therapies that included lenalidomide and a PI and have demonstrated disease progression on or after the last therapy. - in combination with carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma who have received one to three prior lines of therapy. - as monotherapy, in patients who have received at least three prior lines of therapy including a PI and an immunomodulatory agent (IMiD) or who are double-refractory to a PI and an IMiD. DARZALEX® SC in combination with bortezomib, cyclophosphamide and dexamethasone, is indicated for the treatment of newly diagnosed patients with light chain (AL) amyloidosis.
**Contraindications** Patients with a history of severe hypersensitivity to daratumumab or any of the excipients.
L01FC01
daratumumab
Manufacturer Information
JOHNSON & JOHNSON INTERNATIONAL (SINGAPORE) PTE. LTD.
Cilag AG
Active Ingredients
Documents
Package Inserts
Darzalex SC PI.pdf
Approved: May 29, 2023
