Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET
4.2 Dose and method of administration **General Treatment Schedule** The recommended cumulative dose of MAVENCLAD is 3.5 mg/kg body weight over 2 years, administered as 1 treatment course of 1.75 mg/kg per year. Each treatment course consists of 2 treatment weeks, one at the beginning of the first month and one at the beginning of the second month of the respective year. Each treatment week consists of 4 or 5 days on which a patient receives 10 mg or 20 mg (one or two tablets) as a single daily dose, depending on body weight. Patients should receive no more than 2 treatment courses over two consecutive years. The recommended dose should not be exceeded. Following completion of the 2 treatment courses, no further cladribine treatment is required in year 3 and year 4 (refer to 5.1 Pharmacodynamic properties, Clinical efficacy and safety – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Re-initiation of therapy after year 4 has not been studied. **Criteria for Starting and Continuing Therapy** Lymphocyte monitoring Lymphocyte counts must be - normal before initiating MAVENCLAD therapy, - at least 800 cells/mm3 before the second treatment course in year 2. If necessary, the treatment course in year 2 can be delayed for up to 6 months to allow for recovery of lymphocytes. If this recovery takes more than 6 months, the patient should not receive MAVENCLAD anymore. **Dose** **Distribution of dose** The distribution of the total dose over the 2 years of treatment is provided in Table 1. Note that for some weight ranges the number of tablets may vary from one treatment week to the next. 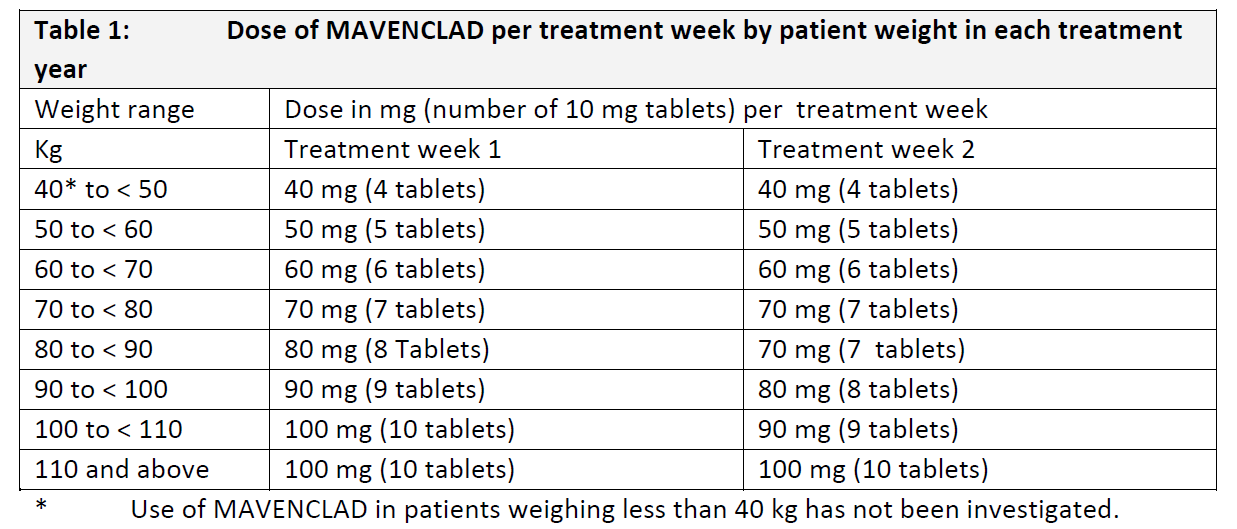 Table 2 shows how the total number of tablets per treatment week is distributed over the individual days. It is recommended that the daily MAVENCLAD doses in each treatment week be taken at intervals of 24 hours at approximately the same time each day. If a daily dose consists of two tablets, both tablets are taken together as a single dose. 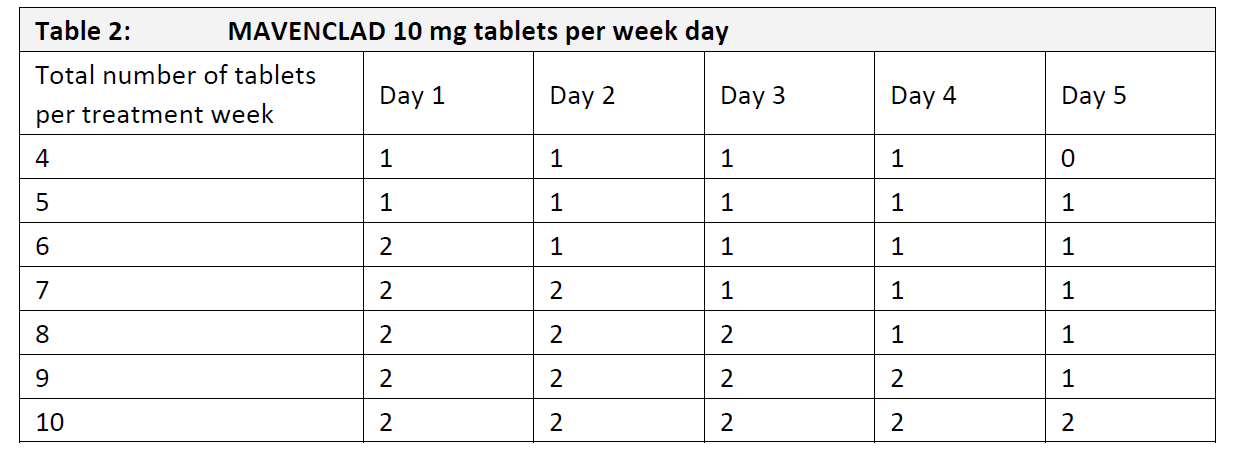 **Dosing Errors** A missed dose can be taken as soon as remembered, if remembered on the same calendar day. A missed dose must not be taken if it is not remembered until the following day. In this case, the patient must take the next dose as scheduled, and extend the number of days in that treatment week. For example, if a patient forgets to take the Day 3 dose and does not remember until Day 4, the Day 3 dose is taken on Day 4, and the total number of days in the treatment week is extended by one day. If two consecutive doses are missed, the same rule applies, and the treatment week is extended by two days. In case of an accidental dose higher than prescribed, the clinical status of the patient must be reviewed and a decision made as to whether and how to continue treatment. **Paediatric population** The safety and efficacy of MAVENCLAD in children aged under 18 years old have not been established. No data are available. **Method of Administration** MAVENCLAD tablets must be taken orally, with water, and swallowed without chewing. It is unlikely that food intake will have a clinically significant effect on absorption of cladribine. Therefore, MAVENCLAD can be taken before or after a meal. As tablets are uncoated, they must be swallowed immediately once removed from the blister and not left exposed on surfaces or handled for any period of time greater than that required for dosing. If a tablet is left on a surface, or if a broken or fragmented tablet is released from the blister, the area must be thoroughly washed afterwards. The patient’s hands must be dry when handling the tablets and washed thoroughly afterwards.
ORAL
Medical Information
4.1 Therapeutic indications MAVENCLAD is indicated for the treatment of relapsing-remitting multiple sclerosis (RRMS) to reduce the frequency of clinical relapses and to delay the progression of physical disability.
4.3 Contraindications - MAVENCLAD is contraindicated in patients with hypersensitivity to cladribine or to any of the tablet excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. - MAVENCLAD therapy must not be initiated in patients who are infected with the human immunodeficiency virus (HIV). - MAVENCLAD must not be initiated in patients with active chronic infections (tuberculosis, hepatitis) (refer to 4.4 Special warnings and precautions for use – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - MAVENCLAD therapy must not be initiated in immunocompromised patients, including patients receiving immunosuppressive or myelosuppressive therapy with agents such as cyclosporin, methotrexate, mitoxantrone, azathioprine, natalizumab, or chronic use of corticosteroids. Acute short-term therapy with corticosteroids can be administered. - MAVENCLAD must not be used in patients with moderate or severe renal impairment (creatinine clearance < 60 mL/min). - MAVENCLAD is contraindicated during pregnancy and breastfeeding (refer to 4.4 Special warnings and precautions for use, 4.6 Fertility, pregnancy and lactation – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - MAVENCLAD is contraindicated in active malignancy.
L04AA40
cladribine
Manufacturer Information
MERCK PTE. LTD.
NerPharMa S.R.L.
Active Ingredients
Documents
Package Inserts
MAVENCLAD TABLET 10MG PI.pdf
Approved: March 23, 2022
