Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION
**DOSAGE AND ADMINISTRATION** **_A. GENERAL_** **Only the lowest dose necessary to obtain adequate visualization should be used.** Use only the recommended concentration for the particular procedure to be undertaken. Patients should be well hydrated prior to and following administration of Optiray (ioversol). Do not dehydrate patients for any procedure. Optiray (ioversol) should be inspected visually for particulate matter and discoloration prior to administration. If either is present the vial should be discarded. Optiray should not be transferred into other delivery systems except immediately before use and should be used immediately once the seal has been punctured. It is advisable that Optiray be at or close to body temperature when injected. Under no circumstances should other drugs be administered concomitantly in the same syringe or i.v. administration set as Optiray because of a potential for chemical incompatibility. Patency of the vessel and the position of the catheter tip or needle should be checked with a small pilot dose of Optiray before injecting the full dose. The catheter tip should be kept free of aspirated blood. Prolonged contact of Optiray with blood must be avoided because of potential thromboembolic complications. The volume of each individual injection is a more important consideration than the total dose used. When large individual volumes are administered, sufficient time should be permitted to elapse between each injection to allow for subsidence of hemodynamic disturbances. Any unused portion of one container should be discarded. **_B. INTRAVASCULAR DOSAGE AND ADMINISTRATION_** **_3\. PERIPHERAL ARTERIOGRAPHY_** Optiray 350 may be used for arteriograms of the lower extremities. **_Patient Preparation_** The procedure is normally performed with local anesthesia. General anesthesia usually is not required (See PRECAUTIONS, General – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **_Precautions_** In addition to the general precautions previously described, moderate decreases in blood pressure occur frequently with intra-arterial injections. This change is usually transient; however, the blood pressure should be monitored for approximately 10 minutes following injection. Injection of Optiray in patients with severe arterial disease (e.g. thromboangiitis obliterans, severe atherosclerosis, ischemia, thrombosis, significant obstruction) should be undertaken with extreme caution and only when absolutely necessary. When injections are being made in the distal aorta for aorto-iliac run-off studies, the possibility of inadvertent injection of a large dose into a branch of the aorta or intramural dissection should be considered. To prevent extravasation or subintimal injection, the position of the catheter tip or needle should be carefully evaluated. Fluoroscopy is recommended. **Pulsation must be present in the artery to be injected.** A small dose of 1 – 2 mL should be administered to locate the exact site of the needle or catheter tip. Great care is necessary to avoid entry of a large bolus into an aortic branch. Severe pain, paresthesia or peripheral muscle spasm during injection may require discontinuance of the procedure and a re-evaluation of the catheter tip or needle placement. Following catheter procedures, gentle pressure hemostasis is advised, followed by observation and immobilization of the limb for several hours to prevent hemorrhage from the site of arterial puncture. **_Adverse Reactions_** Adverse reactions observed during peripheral arteriography may be due to trauma during the procedure or to the injection of the contrast material. Adverse reactions reported with the use of iodinated contrast media include hypotension, soreness in extremities, transient arterial spasm, contrast medium induced thrombosis, embolism, gangrene, perforation of vessels, extravasation, hemorrhage, hematoma formation with tamponade, injury to spinal cord and nerves and other structures in close proximity to the artery; transverse myelitis, thrombosis, dissecting aneurysm, arteriovenous fistula, dislodgment of atheromatous plaques, subintimal injection, leg pain, renal damage including infarction and tubular necrosis due to accidental filling of the renal arteries. **_Usual Adult Dosage_** The usual single adult dose for aorto-iliac run-off studies is 20 – 50 mL; for iliac and femoral arteries 10 – 30 mL. These doses may be repeated as indicated. The total procedural dose should be limited to the smallest volume required to obtain a diagnostic examination and should not usually exceed 250 mL. **_4\. SELECTIVE CORONARY ARTERIOGRAPHY WITH OR WITHOUT LEFT VENTRICULOGRAPHY_** Optiray 350 is recommended for this procedure. **_Precautions_** Since the risk in coronary arteriography is increased if the procedure is performed shortly after acute myocardial infarction, some physicians recommend that this procedure should not be performed for approximately 4 weeks following the diagnosis of myocardial infarction. Mandatory pre-requisites to the procedure are experienced personnel, ECG monitoring apparatus and adequate facilities for immediate resuscitation and cardioversion. Patients should be monitored continuously by ECG and vital signs throughout the procedure. The injection of relatively large volumes of hypertonic solutions (e.g. contrast media) into the heart chambers can cause significant hemodynamic disturbances. Caution is advised especially in patients with incipient heart failure because of the possibility of aggravating the pre-existing condition. Hypotension should be corrected promptly since it may induce serious arrhythmias. **_Adverse Reactions_** Most patients will have transient ECG changes during the procedure. The following adverse effects have occurred in conjunction with the administration of iodinated intravascular contrast agents for this purpose: hypotension, shock, anginal pain, coronary thrombosis, myocardial infarction, cardiac arrhythmias (bradycardia, ventricular tachycardia, heart block, ventricular fibrillation) cardiac arrest and death. Severe adverse reactions, especially arrhythmias, are likely to occur with greater frequency following right coronary artery injection. Fatalities have been reported. Complications to the procedures include dissection of coronary arteries, dislodgement of atheromatous plaques, embolization from the catheter, perforation of heart chambers or coronary arteries with cardiac tamponade, hemorrhage and thrombosis. **_Usual Adult Dosage_** The usual adult dose range with Optiray 350 for left coronary arteriography is 2 – 10 mL and for right coronary arteriography is 2 – 6 mL. For left ventriculography, the usual single adult dose is 30 – 40 mL. These doses may be repeated if indicated; however, several minutes should be allowed to elapse between injections to allow for subsidence of hemodynamic disturbance, and the total procedural dose should be limited to the smallest volume necessary to obtain a diagnostic examination. The total procedural dose should not exceed 250 mL. **_Pediatric Dosage and Administration_** Optiray 350 is recommended for this procedure in children 1 year of age and over. The usual single injection dose of Optiray 350 is 1.25 mL/kg of body weight with a range of 1 mL/kg to 1.5 mL/kg. When multiple injections are given, the total administered dose should not exceed 5 mL/kg up to a total volume of 250 mL. **_5\. AORTOGRAPHY AND VISCERAL ARTERIOGRAPHY_** _Optiray 350 is recommended for this procedure. Great care is necessary to avoid all entry of a large bolus into an aortic branch. Mesenteric necrosis, acute pancreatitis, renal infarction, acute tubular necrosis, renal shutdown and serious neurologic complications, including paraplegia and quadriplegia, have been reported and may be attributable to an excessive dose being injected into an aortic branch or arterial trunks supplying the spinal arteries or to prolonged contact time of the concentrated contrast medium with the CNS tissue. Conditions which can contribute to prolonged contact time include decreased circulation, aortic stenosis or partial occlusions distal to the site of injection, abdominal compression, hypotension, general anesthesia or the administration of vasopressors. When these conditions exist or occur, the necessity of performing or continuing the procedure should be carefully evaluated and the dose and number of repeat injections should be maintained at a minimum with appropriate intervals between injections._ _**_Adverse Reactions_**_ _With aortic injection, depending on the technique employed, the risks of this procedure also include the following: injury to the aorta and neighbouring organs, pleural puncture, renal damage including infarction and acute tubular necrosis with oliguria and anuria due to accidental filling of the renal arteries, retroperitoneal hemorrhage from the translumbar approach and spinal cord injury and pathology associated with the syndrome of transverse myelitis. Occasional serious neurological complications including paraplegia have been reported in patients with aortoiliac or femoral artery obstruction, abdominal compression, hypotension, hypertension, spinal anesthesia and injection of vasopressor drugs to enhance contrast. In such patients, the concentration, volume and number of injections should be kept to a minimum._ _**_Adult Dosage and Administration_**_ _Optiray 350 is recommended for this procedure. The usual individual injection volumes are as follows:_ _abdominal aorta20 – 50 mLsuperior mesenteric artery20 – 40 mLrenal artery4 – 10 mL_ _Total procedural dose should not exceed 250 mL._ _**_6\. INTRAVENOUS CONTRAST ENHANCEMENT IN COMPUTED TOMOGRAPHY (CT)_**_ _Because unenhanced scanning may provide adequate information in the individual patient and the injection of contrast media may obscure certain lesions visible on the plain scan, contrast enhancement is usually performed only if the unenhanced scan has not provided sufficient information. The decision to employ contrast enhancement, which is associated with additional risk and increased radiation exposure, should be based upon a careful evaluation of the patient's clinical condition, renal and cardiac reserve, the status of the blood-brain barrier and other radiological and unenhanced CT findings._ _**_Warnings_**_ _Patients with diabetes mellitus, impaired renal function and congestive heart failure are considered to be at greater risk of developing acute renal failure following injection of the large doses of contrast media required for contrast enhancement in CT scanning. Convulsions and other serious neurologic complications including stroke have occurred in patients with primary or metastatic cerebral lesions or breached blood-brain barrier or slowed cerebral circulation following the administration of iodine-containing radiopaque media for enhancement of CT brain images._ _**_Patient Preparation_**_ _No special patient preparation is required for contrast enhancement in computerized tomography. **However, it has to be insured that patients are well hydrated prior to examination.** In patients undergoing abdominal or pelvic examination, opacification of the bowel by dilute oral contrast medium may be valuable in scan interpretation._ _**_Precautions_**_ _Patient motion, including respiration, can markedly affect image quality, therefore patient cooperation is essential._ _The use of an intravascular contrast medium can obscure some tumours in patients undergoing CT evaluation, resulting in a false negative diagnosis._ _**_Computed Tomography of the Head Neoplastic Conditions_**_ _Optiray 350 may be used to enhance the demonstration of the presence and extent of certain primary or metastatic malignancies._ _The usefulness of contrast enhancement for the investigation of the retrobulbar space and in cases of low grade or infiltrative glioma has not been demonstrated._ _In cases where lesions have calcified, there is less likelihood of enhancement. Following therapy, tumours may show decreased or no enhancement. Maximum contrast enhancement of certain tumours may be delayed necessitating delayed scans._ _**_Non-Neoplastic Conditions_**_ _The use of Optiray 350 may be beneficial in the image enhancement of non-neoplastic lesions, such as cerebral infarctions of recent onset; however, some infarctions are obscured if contrast media are used._ _Arteriovenous malformations and aneurysms will show contrast enhancement. In the case of these vascular lesions, the enhancement is probably dependent on the iodine content of the circulating blood pool._ _**Hematomas and intraparenchymal bleeders seldom demonstrate any contrast enhancement.** However, in cases of intraparenchymal clot, for which there is no obvious clinical explanation, contrast medium administration may be helpful in ruling out the possibility of associated arteriovenous malformation. (Also see Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_)._ _The opacification of the inferior vermis following contrast medium administration has resulted in false positive diagnoses in a number of normal studies._ _**_Usual Adult Dosage_**_ _For adults the usual dosage of Optiray 350 is 50–100 mL. A maximum dose of 150 mL of Optiray 350 should not be exceeded. Scanning is usually performed immediately after injection._ _**_Body Computed Tomography_**_ _Optiray 350 may be administered for contrast enhancement of the organs, tissues and larger blood vessels of the chest, abdomen and pelvis._ _Continuous or multiple scans separated by intervals of 1 – 3 seconds during the first 30 – 90 seconds post-injection of the contrast medium (dynamic CT scanning) are required to demonstrate enhanceable lesions not seen with CT alone. Subsets of patients in whom delayed body CT scans might be helpful have not been identified._ _Inconsistent results have been reported and abnormal and normal tissues are usually isodense during the time frame used for delayed CT scanning. At present, consistent results have been documented using dynamic CT techniques only._ _**_Usual Adult Dosage_**_ _Optiray 350 may be administered by bolus injection, rapid infusion or by a combination of both. Depending on the area to be examined, the usual dose range for infusion is 30 – 100 mL. When prolonged enhancement is required, 25 – 50 mL may be given as a rapid bolus and the remainder as an infusion. The total dose should not exceed 150 mL of Optiray 350. Scanning is usually performed immediately after injection._ _**_7\. VENOGRAPHY_**_ _Optiray 350 may be used to visualize the peripheral venous circulation. Venograms are obtained by injection or infusion into an appropriate vein in the lower extremity._ _**_Precautions_**_ _In addition to the general precautions previously described, specific caution is advised when venography is required in patients with suspected thrombosis, phlebitis, severe ischemic disease, local infection or a significantly obstructed venous system._ _Extreme caution is necessary to avoid extravasation and fluoroscopy is recommended. This is especially important in patients with severe venous disease._ _**_Adverse Reactions_**_ _Complications of the procedure include bleeding, thrombosis, embolism, contrast medium-induced thrombophlebitis, gangrene and major systemic adverse reactions._ _**_Usual Adult Dosage_**_ _The usual adult dose of Optiray 350 will range from 20 – 100 mL for the lower extremity._ _Following the procedure, the venous system should be flushed with normal or heparinized saline solution. Massage and elevation of the leg are also helpful for clearing the contrast medium from the extremity to prevent post-procedural thrombophlebitis. The maximum dose should not usually exceed 250 mL._ _**_8\. EXCRETORY UROGRAPHY_**_ _Optiray 350 may be used for excretory urography. Following intravenous injection in patients with normal renal function, Optiray is excreted mostly by the kidneys. Maximum radiographic density in the calyces and pelves occurs in most instances within 5 to 15 minutes after injection._ _In patients with severe renal impairment, contrast visualization may be substantially delayed, or may not occur at all._ _**_Patient Preparation_**_ _A low residue diet the day preceding the examination, and a laxative the evening before the examination, may be given unless contraindicated. **Partial dehydration is dangerous and may contribute to acute renal failure**. Maintenance of normal hydration is desirable._ _**_Precautions_**_ _Adequate renal function must be present. Dehydration will not improve contrast quality in patients with impaired renal function and will increase the risk of contrast induced renal damage. The examination should not be repeated for at least 72 hours because of the potential of additive renal damage. (Also see WARNINGS and PRECAUTIONS – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.)_ _**_Adverse Reactions_**_ _All adverse reactions known to occur with the i.v. use of Optiray can also occur with excretory urography (see Adverse Reactions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_)._ _**_Usual Adult Dosage_**_ _The usual adult dose of Optiray 350 is 50 mL in the average normal adult. The dose is injected intravenously, usually within 1 – 3 minutes. Maximum dose of 140 mL of Optiray 350 should not be exceeded._ _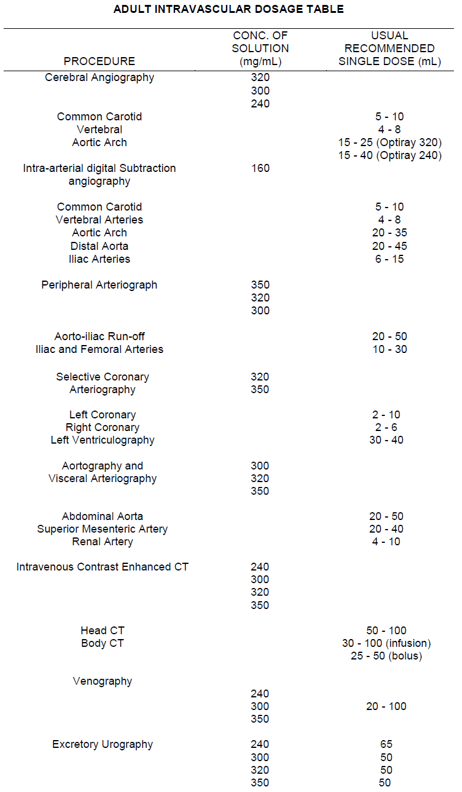_ _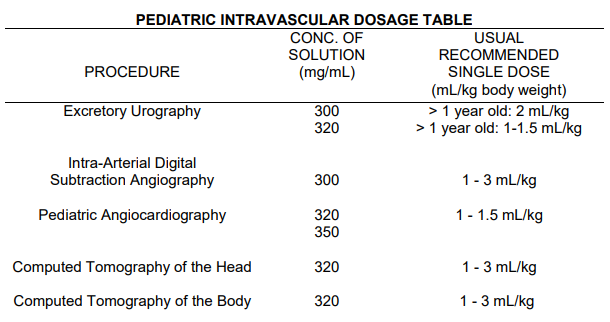_
INTRAVASCULAR
Medical Information
**INDICATIONS AND CLINICAL USES** **A. _INTRAVASCULAR_** **_Adults_** Optiray 350 (ioversol 350 mgI/mL) is recommended in adults for coronary arteriography and ventriculography, peripheral and visceral arteriography, intravenous contrast enhancement in computed tomography of the head and body, excretory urography, intravenous digital subtraction angiography and venography. **_Pediatric_** Optiray 350 is indicated in children for angiocardiography.
**CONTRAINDICATIONS** Optiray (ioversol) should not be administered to patients with known or suspected hypersensitivity to ioversol or in cases of clinically significant impairment of both hepatic and renal function.
V08AB07
ioversol
Manufacturer Information
TRANSMEDIC PTE LTD
Liebel-Flarsheim Company LLC
Liebel-Flarsheim Canada Inc.
Active Ingredients
Documents
Package Inserts
Optiray 350 Injection 74% PI (Raleigh).pdf
Approved: June 21, 2022
