Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
CAPSULE
**4.2 Posology and method of administration** **General** Gabapentin is given orally with or without food. When, in the judgment of the clinician, there is a need for dose reduction, discontinuation, or substitution with an alternative medication, this should be done gradually over a minimum of 1 week. **Epilepsy** _Adults and pediatric patients older than 12 years of age_ In clinical trials, the effective dosing range was 900 mg/day to 1800 mg/day. Therapy may be initiated by administering 300 mg three times a day on Day 1, or by titrating the dose (Table 1). Thereafter, the dose can be increased in three equally divided doses up to a maximum dose of 1800 mg/day. Doses up to 2400 mg/day have been well tolerated in long-term open-label clinical studies. Doses up to 3600 mg/day have been administered to a small number of patients for a relatively short duration, and have been well tolerated. The maximum time between doses in the three times a day schedule should not exceed 12 hours to prevent breakthrough convulsions. 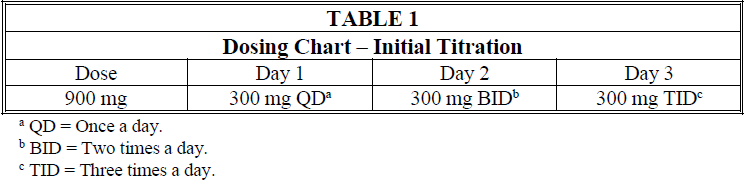 _Pediatric patients aged 3 to 12 years_ The starting dose should range from 10 to 15 mg/kg/day given in equally divided doses (three times a day), and the effective dose reached by upward titration over a period of approximately 3 days. The effective dose of gabapentin in pediatric patients aged 5 years and older is 25 to 35 mg/kg/day given in equally divided doses (three times a day). The effective dose in pediatric patients aged 3 to less than 5 years is 40 mg/kg/day given in equally divided doses (three times a day). Doses up to 50 mg/kg/day have been well tolerated in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. It is not necessary to monitor gabapentin plasma concentrations to optimize gabapentin therapy. Further, gabapentin may be used in combination with other anti-epileptic drugs without concern for alteration of the plasma concentrations of gabapentin or serum concentrations of other anti-epileptic drugs. **Neuropathic pain in adults** The starting dose is 900 mg/day given in three equally divided doses, and increased if necessary, based on response, up to a maximum dose of 3600 mg/day. Therapy should be initiated by titrating the dose (Table 1). **Dose adjustment in impaired renal function in patients with neuropathic pain or epilepsy** Dose adjustment is recommended in patients with compromised renal function (Table 2) and/or in those undergoing hemodialysis. 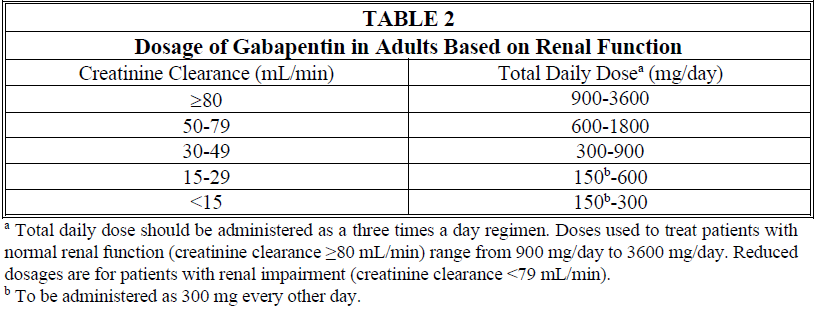 **Dose adjustment in patients undergoing hemodialysis** For patients undergoing hemodialysis who have never received gabapentin, a loading dose of 300 mg to 400 mg is recommended, and then 200 mg to 300 mg of gabapentin following each 4 hours of hemodialysis.
ORAL
Medical Information
**4.1 Therapeutic indications** **Epilepsy** Gabapentin is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 3 years and older. Safety and effectiveness for adjunctive therapy in pediatric patients younger than 3 years have not been established (see Section **4.2 Posology and method of administration** – _Pediatric patients aged 3 to 12 years_). **Neuropathic pain** Gabapentin is indicated for the treatment of neuropathic pain which include diabetic pain, post-herpetic neuralgia and trigeminal neuralgia. Safety and effectiveness in patients younger than 18 years have not been established.
**4.3 Contraindications** Gabapentin is contraindicated in patients who are hypersensitive to gabapentin or the product's components.
N03AX12
xn 03 ax 12
Manufacturer Information
VIATRIS PRIVATE LIMITED
Pfizer Manufacturing Deutschland GmbH
Pfizer Pharmaceuticals LLC
Active Ingredients
Documents
Package Inserts
NEURONTIN CAPSULES PI.pdf
Approved: October 14, 2022
