Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION
**4.2 Posology and method of administration** Soliqua is available in two pre-filled pens, providing different dosing options, i.e. Soliqua (10–40) pen, Soliqua (30–60) pen respectively. The differentiation between the pen strengths is based on the dose range of the pen. - Soliqua 100 units/ml + 50 micrograms/ml pre-filled pen delivers dose steps from **10–40 units** of insulin glargine in combination with **5–20 mcg** lixisenatide (Soliqua (10–40) pen). - Soliqua 100 units/ml + 33 micrograms/ml pre-filled pen delivers dose steps from **30–60 units** of insulin glargine in combination with **10–20 mcg** lixisenatide (Soliqua (30–60) pen). To avoid medication errors, the prescriber must make sure that the correct strength and number of dose steps is stated in the prescription (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Posology The dose must be individualised based on clinical response and is titrated based on the patient’s need for insulin. The lixisenatide dose is increased or decreased along with insulin glargine dose and also depends on which pen is used. _Starting dose_ Therapy with basal insulin or glucagon-like peptide-1 (GLP-1) receptor agonist or oral glucose lowering medicinal product other than metformin and SGLT-2 inhibitors should be discontinued prior to initiation of Soliqua. The starting dose of Soliqua is based on previous anti-diabetic treatment, and in order not to exceed the recommended lixisenatide starting dose of 10 mcg: 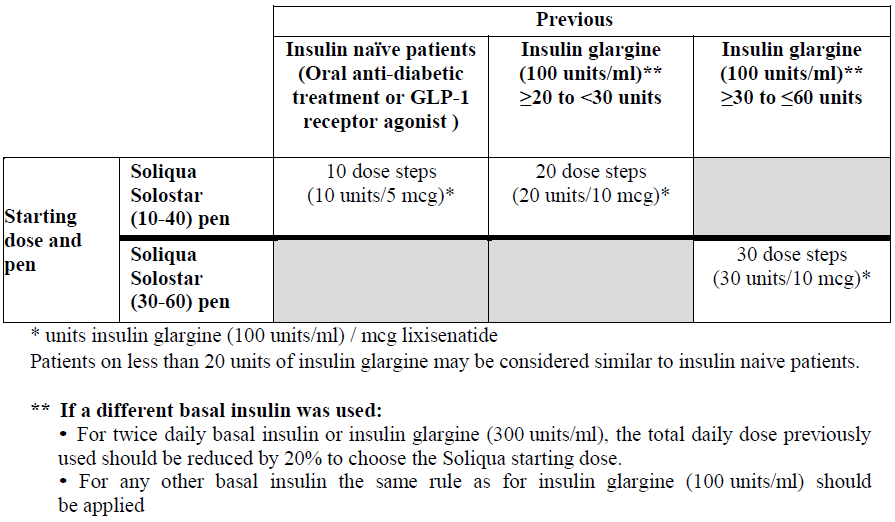 The maximum daily dose is 60 units insulin glargine and 20 mcg lixisenatide corresponding to 60 dose steps. Soliqua should be injected once a day within one hour prior to a meal. It is preferable that the prandial injection is performed before the same meal every day, when the most convenient meal has been chosen. _Dosage titration_ Soliqua is to be dosed in accordance with the individual patient's need for insulin. It is recommended to optimise glycaemic control via dose adjustment based on fasting plasma glucose (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Close glucose monitoring is recommended during the transfer and in the following weeks. - If the patient starts with the Soliqua (10–40) pen, the dose may be titrated up to 40 dose steps with this pen. - For doses >40 dose steps/day titration must be continued with Soliqua (30–60) pen. - If the patient starts with the Soliqua (30–60) pen, the dose may be titrated up to 60 dose steps with this pen. - For total daily doses >60 dose steps/day, Soliqua must not be used. Patients adjusting the amount or timing of dosing should only do so under medical supervision with appropriate glucose monitoring (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _Missed dose_ If a dose of Soliqua is missed, it should be injected within the hour prior to the next meal. _Special population_ _Elderly (≥65 years old)_ Soliqua can be used in elderly patients. The dose should be adjusted on an individual basis, based on glucose monitoring. In the elderly, progressive deterioration of renal function may lead to a steady decrease in insulin requirements. For lixisenatide no dose adjustment is required based on age. The therapeutic experience of Soliqua in patients ≥75 years of age is limited. _Renal impairment_ Soliqua is not recommended in patients with severe renal impairment and end-stage renal disease as there is no sufficient therapeutic experience with use of lixisenatide. No dose adjustment is required for lixisenatide in patients with mild or moderate renal impairment. In patients with renal impairment, insulin requirements may be diminished due to reduced insulin metabolism. In patients with mild to moderate renal impairment using Soliqua, frequent glucose monitoring and dose adjustment may be necessary. _Hepatic impairment_ No dose adjustment of lixisenatide is needed in patients with hepatic impairment (see section 5.2 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). In patients with hepatic impairment, insulin requirements may be diminished due to reduced capacity for gluconeogenesis and reduced insulin metabolism. Frequent glucose monitoring and dose adjustment may be necessary for Soliqua in patients with hepatic impairment. _Paediatric population_ There is no relevant use of Soliqua in the paediatric population. Method of administration Soliqua is to be injected subcutaneously in the abdomen, deltoid, or thigh. The injection sites should be rotated within the same region (abdomen, deltoid, or thigh) from one injection to the next in order to reduce the risk of lipodystrophy and cutaneous amyloidosis (see section 4.4 and 4.8 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Patients should be instructed to always use a new needle. The re-use of insulin pen needles increases the risk of blocked needles, which may cause under- or overdosing. In the event of blocked needles, patients must follow the instructions described in the Instructions for Use accompanying the package leaflet (see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Soliqua must not be drawn from the cartridge of the pre-filled pen into a syringe to avoid dosing errors and potential overdose (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
SUBCUTANEOUS
Medical Information
**4.1 Therapeutic indications** Soliqua is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus to improve glycaemic control as an adjunct to diet and exercise in addition to metformin with or without SGLT-2 inhibitors. (For study results with respect to effect on glycaemic control, and the populations studied, see section 4.4 and 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3 Contraindications** Hypersensitivity to the active substances or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_.
A10AE54
insulin glargine and lixisenatide
Manufacturer Information
SANOFI-AVENTIS SINGAPORE PTE. LTD.
Sanofi-Aventis Deutschland GmbH
Active Ingredients
Documents
Package Inserts
Soliqua Solution for Injection in a Pre-filled Pen 100 units per ml + 33 mcg per ml PI and IFU.pdf
Approved: May 7, 2021
