Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INFUSION, SOLUTION CONCENTRATE
**4.2 Posology and method of administration** SOLIRIS must be administered by a healthcare professional and under the supervision of a physician experienced in the management of patients with haematological and/or renal disorders. **Posology** **_Adult patients_** _**In Paroxysmal Nocturnal Haemoglobinuria (PNH):**_ The PNH dosing regimen for adult patients (≥18 years of age) consists of a 4-week initial phase followed by a maintenance phase: - Initial phase: 600 mg of SOLIRIS administered via a 25 – 45-minute intravenous infusion every week for the first 4 weeks. - Maintenance phase: 900 mg of SOLIRIS administered via a 25 – 45-minute intravenous infusion for the fifth week, followed by 900 mg of SOLIRIS administered via a 25 – 45-minute intravenous infusion every 14 ± 2 days (see section 5.1). _**In Atypical Haemolytic Uremic Syndrome (aHUS):**_ The aHUS dosing regimen for adult patients (≥ 18 years of age) consists of a 4-week initial phase followed by a maintenance phase: - Initial phase: 900 mg of SOLIRIS administered via a 25 – 45-minute intravenous infusion every week for the first 4 weeks. - Maintenance phase: 1,200 mg of SOLIRIS administered via a 25 – 45-minute intravenous infusion for the fifth week, followed by 1,200 mg of SOLIRIS administered via a 25 – 45-minute intravenous infusion every 14 ± 2 days (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). _**Paediatric patients in PNH and aHUS:**_ Paediatric PNH and aHUS patients with body weight ≥ 40kg are treated with the adult dosing recommendations, respectively. In paediatric PNH and aHUS patients with body weight below 40 kg, the SOLIRIS dosing regimen consists of: 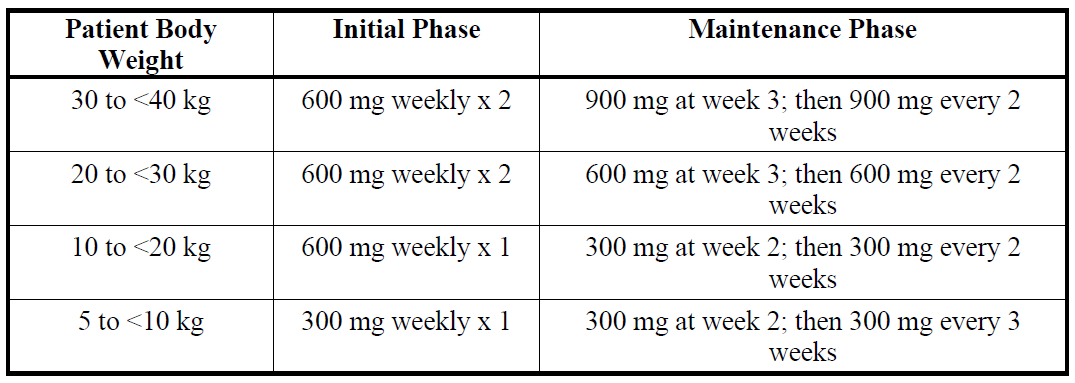 SOLIRIS has not been studied in patients with PNH who weigh less than 40kg. The posology of SOLIRIS for PNH patients less than 40kg weight is based on the posology used for patients with aHUS and who weigh less than 40kg. For adults and paediatric aHUS patients supplemental dosing of SOLIRIS is required in the setting of concomitant PE/PI (plasmapheresis or plasma exchange, or fresh frozen plasma infusion): 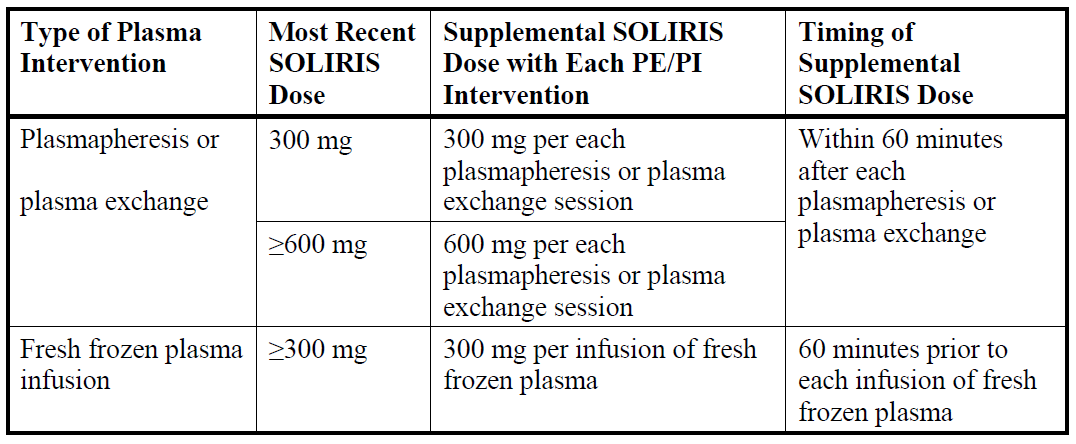 **Treatment Monitoring** aHUS patients should be monitored for signs and symptoms of thrombotic microangiopathy (TMA) (refer to section 4.4 aHUS Laboratory Monitoring – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). SOLIRIS treatment is recommended to continue for the patient’s lifetime, unless the discontinuation of SOLIRIS is clinically indicated (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Method of administration** Do not administer as an intravenous push or bolus injection. SOLIRIS should only be administered via intravenous infusion as described below. For instructions on dilution of the medicinal product before administration, see section 6.6 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. The diluted solution of SOLIRIS should be administered by intravenous infusion over 25 – 45 minutes in adults and 1–4 hours in paediatric patients via gravity feed, a syringe-type pump, or an infusion pump. It is not necessary to protect the diluted solution of SOLIRIS from light during administration to the patient. Patients should be monitored for one hour following infusion. If an adverse event occurs during the administration of SOLIRIS, the infusion may be slowed or stopped at the discretion of the physician. If the infusion is slowed, the total infusion time may not exceed two hours in adults and adolescents (aged 12 years to under 18 years) and four hours in children aged less than 12 years. **Elderly** SOLIRIS may be administered to patients aged 65 years and over. There is no evidence to suggest that any special precautions are needed when older people are treated – although experience with SOLIRIS in this patient population is still limited. **Renal impairment** No dose adjustment is required for patients with renal impairment (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). **Hepatic impairment** The safety and efficacy of SOLIRIS have not been studied in patients with hepatic impairment.
INTRAVENOUS
Medical Information
**4.1 Therapeutic indications** SOLIRIS is indicated in adults and children for the treatment of patients with; - Paroxysmal nocturnal haemoglobinuria (PNH). Evidence of clinical benefit is demonstrated in patients with haemolysis with clinical symptom(s) indicative of high disease activity, regardless of transfusion history (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). - Atypical Haemolytic Uremic Syndrome (aHUS) (see section 5.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_).
**4.3 Contraindications** Hypersensitivity to eculizumab, murine proteins or to any of the excipients listed in section 6.1 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_. SOLIRIS therapy must not be initiated in patients (see section 4.4 – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_): - with unresolved _Neisseria meningitidis_ infection. - who are not currently vaccinated against _Neisseria meningitidis_ unless they receive prophylactic treatment with appropriate antibiotics until 2 weeks after vaccination.
L04AA25
xl 04 aa 25
Manufacturer Information
ASTRAZENECA SINGAPORE PTE LTD
Alexion Pharma International Operations Limited (AAMF)
PATHEON ITALIA S.P.A
Active Ingredients
Documents
Package Inserts
Soliris Concentrate for Solution for Infusion_PI.pdf
Approved: May 17, 2023
