Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**Dosage and administration** **Adults with normal renal function (GFR ≥ 90ml/min)** **The recommended dose is 2.5/500 mg, 2.5/ 850 mg or 2.5/1000 mg twice daily.** **The dosage should be individualised on the basis of the patient’s current regimen, effectiveness, and tolerability. Maximum recommended daily dose of TRAJENTA DUO is 5 mg of linagliptin and 2000 mg of metformin (see table 4 for additional dosing information** – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ **).** TRAJENTA DUO should be given with meals to reduce the gastrointestinal undesirable effects associated with metformin. For patients currently not treated with metformin For patients currently not treated with metformin, the recommended starting dose is 2.5 mg linagliptin/500mg metformin hydrochloride twice daily. For patients inadequately controlled on maximal tolerated dose of metformin monotherapy **For patients not adequately controlled on metformin alone, the usual starting dose of TRAJENTA DUO should provide linagliptin dosed as 2.5 mg twice daily (5 mg total daily dose) plus the dose of metformin already being taken.** For patients switching from co-administration of linagliptin and metformin **For patients switching from co-administration of linagliptin and metformin to the fixed dose combination, TRAJENTA DUO should be initiated at the dose of linagliptin and metformin already being taken.** For patients inadequately controlled on dual combination therapy with the maximal tolerated dose of metformin and a sulphonylurea **The dose of TRAJENTA DUO should provide linagliptin dosed as 2.5 mg twice daily (5 mg total daily dose) and a dose of metformin similar to the dose already being taken.** **When TRAJENTA DUO is used in combination with a sulphonylurea, a lower dose of the sulphonylurea may be required to reduce the risk of hypoglycaemia** (see section Special warnings and precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For patients inadequately controlled on dual combination therapy with insulin and the maximal tolerated dose of metformin **The dose of TRAJENTA DUO should provide linagliptin dosed as 2.5 mg twice daily (5 mg total daily dose) and a dose of metformin similar to the dose already being taken.** **When TRAJENTA DUO is used in combination with insulin, a lower dose of insulin may be required to reduce the risk of hypoglycaemia** (see section Special warnings and precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). For the different doses on metformin, TRAJENTA DUO is available in strengths of 2.5 mg linagliptin plus 500 mg metformin hydrochloride, 850 mg metformin hydrochloride or 1000 mg metformin hydrochloride. **Renal insufficiency** A GFR should be assessed before initiation of treatment with metformin containing products and at least annually thereafter. In patients at an increased risk of further progression of renal impairment and in the elderly, renal function should be assessed more frequently, e.g. every 3–6 months. Factors that may increase the risk of lactic acidosis (see Warnings and Precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) should be reviewed before considering initiation of metformin in patients with GFR<60 ml/min. 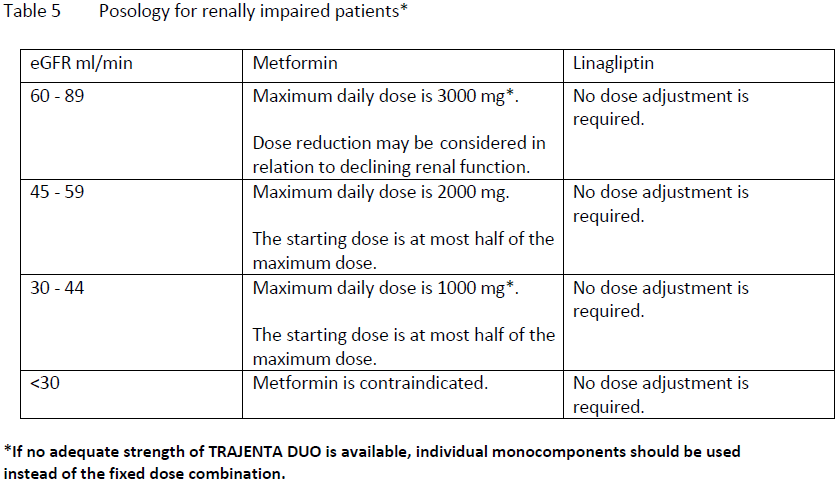 **Hepatic impairment** TRAJENTA DUO is contraindicated in patients with hepatic impairment due to the metformin component (see section Contraindications). **Elderly** As metformin is excreted via the kidney, and elderly patients have a tendency for decreased renal function, **elderly patients taking TRAJENTA DUO should have their renal function monitored regularly** (see section Special warnings and precaution – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_). Experience in patients older than >75 years of age is limited. **Children and adolescents** TRAJENTA DUO is not recommended for use in children below 18 years due to lack of data on safety and efficacy. **Missed dose** If a dose is missed, it should be taken as soon as the patient remembers. However, a double dose should not be taken at the same time. In that case the missed dose should be skipped.
ORAL
Medical Information
**Indications** TRAJENTA DUO is indicated as an adjunct to diet and exercise to improve glycaemic control in adults with type 2 diabetes mellitus when treatment with both linagliptin and metformin is appropriate \[ _see Dosage and Administration and Clinical Studies_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\].
**Contraindications** - **Hypersensitivity to active ingredients linagliptin and/or metformin hydrochloride or to any of the excipients** - **Any type of acute metabolic acidosis (such as lactic acidosis, diabetic ketoacidosis)** - **Diabetic pre-coma** - Severe renal failure (CrCl < 30 mL/min or eGFR < 30 ml/min/1.73m2) - **Acute conditions with the potential to alter renal function** such as: dehydration, severe infection, shock, intravascular administration of iodinated contrast agents (see section Special warnings and precautions – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_) - **Disease which may cause tissue hypoxia (especially acute disease, or worsening of chronic disease)** such as: decompensated heart failure, cardiac or respiratory failure, recent myocardial infarction, shock - **Hepatic impairment** - **Acute alcohol intoxication** - **Alcoholism**
A10BD11
metformin and linagliptin
Manufacturer Information
BOEHRINGER INGELHEIM SINGAPORE PTE. LTD.
BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG
Boehringer Ingelheim Promeco S.A. de C.V. (BI Promeco MX)
Active Ingredients
Documents
Package Inserts
Trajenta Duo PI.pdf
Approved: February 20, 2023
