Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**4.2 Posology and method of administration** For patients weighing less than 47 kg or patients who are unable to swallow Ursofalk® 500mg film-coated tablets, Ursofalk® capsules or suspension are available. The following daily dose is recommended for the various indications: For dissolution of cholesterol gallstones Approx. 10 mg of ursodeoxycholic acid per kg of body weight, equivalent to: up to 60 kg 1 film-coated tablet 61–80 kg 1½ film-coated tablets 81–100 kg 2 film-coated tablets over 100 kg 2½ film-coated tablets The film-coated tablets should be swallowed whole with some liquid in the evening at bedtime. They must be taken regularly. The time required for the dissolution of gallstones is generally 6–24 months. If there is no reduction in the size of the gallstones after 12 months, the therapy should not be continued. The success of the treatment should be checked by means of ultrasound or X-ray examination every 6 months. At the follow-up examinations, a check should be made to see whether calcification of the stones has occurred in the meantime. Should this be the case, the treatment must be ended. For the symptomatic treatment of primary biliary cirrhosis (PBC) The daily dose depends on body weight and ranges from 1½ to 3½ film-coated tablets (14 ± 2 mg of ursodeoxycholic acid per kg of body weight). For the first 3 months of treatment, Ursofalk® 500mg film-coated tablets should be taken divided over the day. When the liver function parameters improve, the daily dose may be taken once daily in the evening. 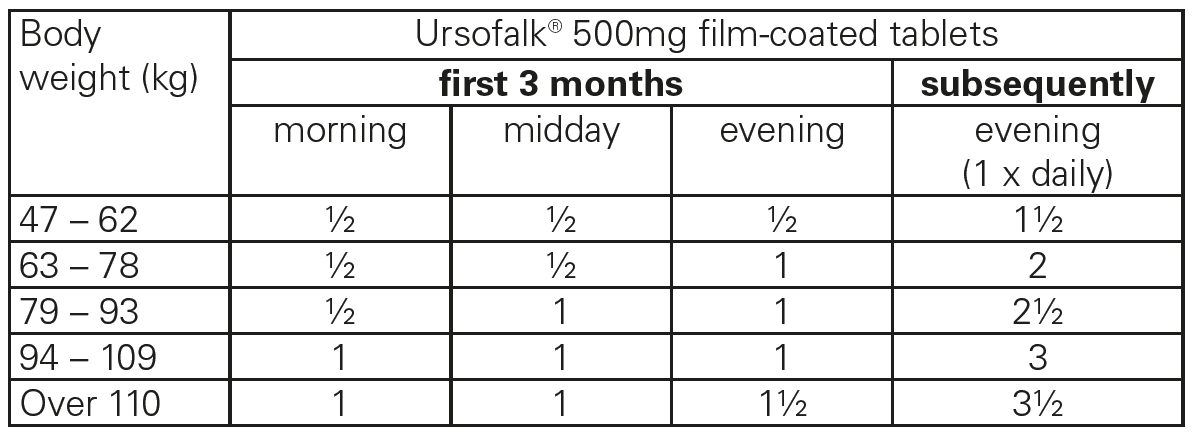 The film-coated tablets should be swallowed whole with some liquid. They must be taken regularly. The use of Ursofalk® 500mg film-coated tablets in primary biliary cirrhosis may be continued indefinitely. In patients with primary biliary cirrhosis, in rare cases the clinical symptoms may worsen at the beginning of treatment, e.g. the itching may increase. In this event, therapy should first be continued with half an Ursofalk® 500mg film-coated tablet or one Ursofalk® capsule (containing 250 mg of ursodeoxycholic acid) daily, and the dose then gradually increased (weekly increase of the daily dose by half a film-coated tablet or one Ursofalk® capsule) until the dose indicated in the respective dosage regimen is reached again. In this case the dose of Ursofalk® should be reduced to one Ursofalk® 250mg capsule daily and then gradually increased again until the dose indicated in the respective dosage regimen is reached again.
ORAL
Medical Information
**4.1 Therapeutic indications** For the dissolution of cholesterol gallstones in the gall bladder. The gallstones must not show as shadows on X-ray images and should not exceed 15 mm in diameter. Gall bladder must be functioning, despite the gallstones. For the symptomatic treatment of primary biliary cirrhosis (PBC), in patients without decompensated hepatic cirrhosis.
**4.3 Contraindications** Ursofalk® 500mg film-coated tablets should not be used in patients with: - acute inflammation of the gall bladder or biliary tract - occlusion of the biliary tract (occlusion of the common bile duct or cystic duct) - frequent episodes of biliary colic - radio-opaque calcified gallstones - impaired contractility of the gall bladder - hypersensitivity to bile acids or any excipient of the formulation
A05AA02
ursodeoxycholic acid
Manufacturer Information
DCH AURIGA SINGAPORE
Losan Pharma GmbH
Losan Pharma GmbH (primary and secondary packager)
Active Ingredients
Documents
Package Inserts
Ursofalk 500mg Tab PI.pdf
Approved: September 21, 2022
