Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
TABLET, FILM COATED
**2 DOSAGE AND ADMINISTRATION** **2.1 Recommended Dose** The recommended dose of TRUVADA is one tablet (containing 200 mg of emtricitabine and 300 mg of tenofovir DF) once daily taken orally with or without food. **2.2 Dose Adjustment for Renal Impairment** Significantly increased drug exposures occurred when emtricitabine or tenofovir were administered to subjects with moderate to severe renal impairment _\[See Warnings and Precautions (5.3) and Clinical Pharmacology (11.2)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. Therefore, adjust the dosing interval of TRUVADA in patients with baseline creatinine clearance 30–49 mL/min using the recommendations in Table 1. These dosing interval recommendations are based on modeling of single-dose pharmacokinetic data in non-HIV infected subjects. The safety and effectiveness of these dosing interval adjustment recommendations have not been clinically evaluated in patients with moderate renal impairment, therefore clinical response to treatment and renal function should be closely monitored in these patients _\[See Warnings and Precautions (5.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. No dose adjustment is necessary for patients with mild renal impairment (creatinine clearance 50–80 mL/min). 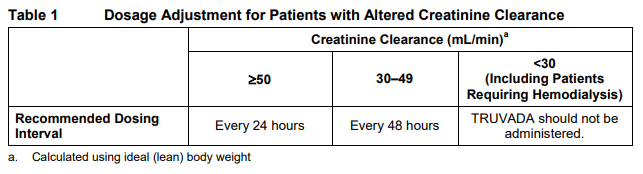 Routine monitoring of estimated creatinine clearance, serum phosphorus, urine glucose, and urine protein should be performed in all individuals with mild renal impairment _\[See Warnings and Precautions (5.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
ORAL
Medical Information
**1 INDICATIONS AND USAGE** TRUVADA®, a combination of emtricitabine and tenofovir disoproxil fumarate (tenofovir DF) is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults. The demonstration of the benefit of the combination emtricitabine and tenofovir DF in antiretroviral therapy is based solely on studies performed in treatment-naïve patients _\[See Clinical Studies (13)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. The following points should be considered when initiating therapy with TRUVADA for the treatment of HIV-1 infection: - It is not recommended that TRUVADA be used as a component of a triple nucleoside regimen. - TRUVADA should not be coadministered with COMPLERA®, STRIBILD®, VIREAD®, GENVOYA®, DESCOVY® , VEMLIDY® or products containing tenofovir alafenamide, tenofovir DF, emtricitabine and lamivudine _\[See Warnings and Precautions (5.4)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_. - In treatment experienced patients, the use of TRUVADA should be guided by laboratory testing and treatment history _\[See Clinical Pharmacology (11.3)_ – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_ _\]_.
**4 CONTRAINDICATIONS** None.
J05AR03
tenofovir disoproxil and emtricitabine
Manufacturer Information
GILEAD SCIENCES SINGAPORE PTE. LTD.
Takeda GmbH
Gilead Sciences Ireland UC
Active Ingredients
Documents
Package Inserts
Truvada Package Insert.pdf
Approved: December 17, 2019
