Regulatory Information
HSA regulatory responsibility and product classification details
Regulatory Responsibility
Product Classification
Formulation Information
INJECTION, SOLUTION (RADIOPHARMACEUTICAL)
**DOSAGE AND ADMINISTRATION** A) **Radiation Safety – Drug Handling** - Wear waterproof gloves and effective shielding when handling Sodium Fluoride F18 Injection 10–200mCi/ml. Use appropriate safety measures, including shielding, consistent with proper patient management to avoid unnecessary radiation exposure to the patient, occupational workers, clinical personnel, and other persons. - Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclide, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclide. - Use aseptic technique to maintain sterility during all operations involved in the manipulation and administration of Sodium Fluoride F18 Injection 10–200mCi/ml. - The dose of Sodium Fluoride F18 Injection 10–200mCi/ml should be minimized consistent with the objectives of the procedure, and nature of the radiation detection devices employed. - The final dose for the patient should be calculated using proper decay factors from the time of End of Synthesis (EOS), and measured by a suitable radioactivity calibration system before administration. \[See Description–Physical characteristics – _please refer to the Product Insert/Patient Information Leaflet published on HSA for the full drug information_\] B) **Radiation Safety – Patient Preparation** - To minimize the radiation-absorbed dose to the bladder, encourage adequate hydration. Encourage the patient to ingest at least 500ml of fluid immediately prior and subsequent to the administration of Sodium Fluoride F18 Injection 10–200mCi/ml. - Encourage the patient to void one-half hour after administration of Sodium Fluoride F18 Injection 10–200mCi/ml and as frequent thereafter as possible for the next 12 hours. C) **Drug Preparation and Administration** - Calculate the necessary volume to administer based on calibration time and dose. - Inspect Sodium Fluoride F18 Injection 10–200mCi/ml visually for particulate matters and discolouration before administration, whenever solution and container permit. - Do not administer Sodium Fluoride F18 Injection 10–200mCi/ml containing particulate matters or discolouration; dispose of these unacceptable or unused preparations in a safe manner, in compliance with applicable regulations. - Aseptically withdraw Sodium Fluoride F18 Injection 10–200mCi/ml from its container. D) **Recommended Dose for Adults** Administer 300–450 MBq (8–12 mCi) as an intravenous injection. E) **Recommended Dose for Paediatric Patients** In reported clinical experience in approximately 100 children, weight-based doses (2.1 MBq/kg) ranging from 19 MBq – 148 MBq (0.5 mCi–4 mCi) were used. F) **Radiation Dosimetry** The age/weight-based estimated absorbed radiation doses (mGy/ MBq) from intravenous injection of Sodium Fluoride F18 Injection are shown in Table 1. These estimates were calculated based on human data and using the data published by the Nuclear Regulatory Commission \[1\] and the International commission on radiological Protection for Sodium Fluoride F18 Injection \[2\]. The bone, bone marrow and urinary bladder are considered target and critical organs. 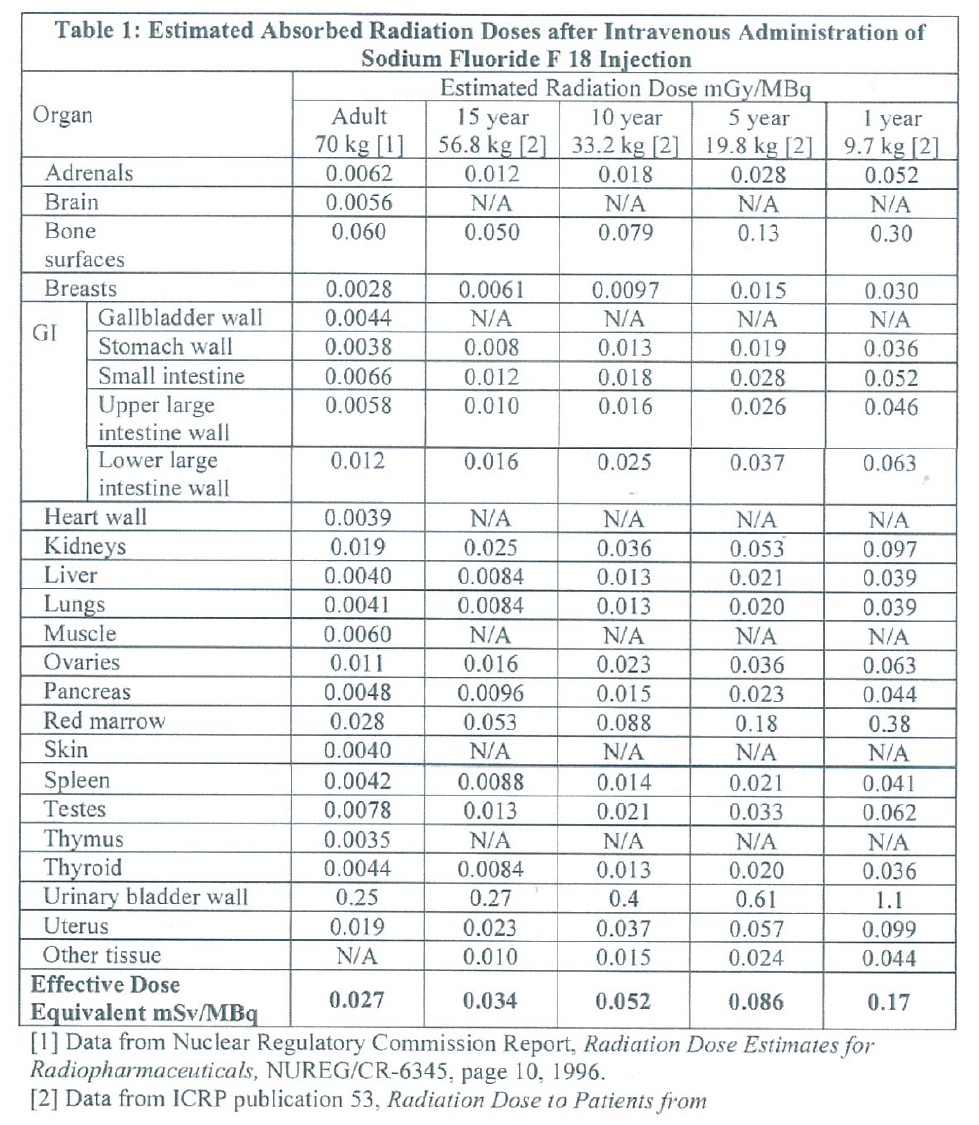 G) **Imaging Guidelines** - Imaging of Sodium Fluoride F18 Injection 10–200mCi/ml can begin 1–2 hours after administration; optimally at 1 hour post administration. - Encourage the patient to void immediately prior to imaging the fluoride F18 radioactivity in the lumbar spine or bony pelvis.
INTRAVENOUS
Medical Information
**INDICATION AND USAGE** Sodium Fluoride F18 Injection 10–200mCi/ml is indicated for diagnostic positron emission tomography (PET) imaging of bone to define areas of altered osteogenic activity.
**CONTRAINDICATIONS** None.
V09IX06
sodium fluoride (18F)
Manufacturer Information
SINGAPORE RADIOPHARMACEUTICALS PTE. LTD.
SINGAPORE RADIOPHARMACEUTICALS PTE. LTD.
Active Ingredients
Documents
Package Inserts
Sodium Fluoride F18 Injection PI.pdf
Approved: November 11, 2013
